当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectra and Photorelaxation of Hydroxyphenyl-benzotriazole-Type UV Absorbers: From Monomers to Nanoparticles.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.jpca.9b09883 Sergej Naumov 1 , Bernd Herzog 2 , Bernd Abel 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.jpca.9b09883 Sergej Naumov 1 , Bernd Herzog 2 , Bernd Abel 1
Affiliation
|
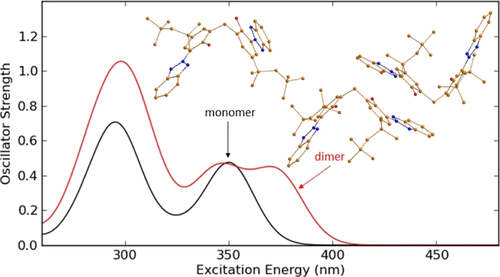
Water-insoluble organic UV filters such as 2,2'-methylene-bis-(6-(2H-benzotriazole-2-yl)-4-(1,1,3,3-tetramethylbutyl)-phenol) (MBBT) can be prepared as aqueous dispersions of nanoparticles. The particles consist of the respective UV absorber molecules and show strong UV absorbance. Because there is a certain solubility of such UV absorbers in organic solvents, it is possible to measure the absorbance spectrum in solution also, for instance, in ethanol or dioxane. The UV spectrum of the aqueous dispersion shows a significant bathochromic shift of the long-wavelength band with an additional shoulder. For the understanding of the observed changes of UV-vis spectra of this UV absorber, either dissolved in an organic solvent or dispersed as nanoparticles in water, density functional theory (DFT) calculations were carried out with the respective monomer and aggregates of MBBT molecules in different media. The calculated UV-vis spectra of isolated, that means dissolved, MBBT molecules in ethanol and in dioxane agree well with the experimentally observed ones. The observed changes in the shape and position of experimental UV-vis spectra in aqueous dispersion cannot be explained with the solvent effect alone. It was found that the studied molecules could form stable energetically favorable π-stacked dimers, which show UV-vis spectra in reasonable agreement with those experimentally observed in aqueous dispersion. Such aggregates of MBBT are most likely the reason for the observed bathochromic shift in the UV-vis absorption spectrum. In addition, the mechanism of the photochemical deactivation of the excited MBBT molecules was studied in detail with time-dependent DFT in dioxane and in water. The energetically most favorable pathway for the deactivation of absorbed energy by MBBT occurs through intramolecular enol-keto tautomerization in the first excited singlet state.
中文翻译:

羟苯基-苯并三唑型紫外线吸收剂的光谱和光弛豫:从单体到纳米粒子。
水不溶性有机UV过滤剂,例如2,2'-亚甲基双-(6-(2H-苯并三唑-2-基)-4-(1,1,3,3-四甲基丁基)-苯酚)(MBBT)可以制备为纳米颗粒的水分散体。颗粒由各自的紫外线吸收剂分子组成,并表现出较强的紫外线吸收率。因为这种紫外线吸收剂在有机溶剂中具有一定的溶解度,所以也可以测量溶液在例如乙醇或二恶烷中的吸收光谱。水性分散体的紫外光谱显示出长波长带具有明显的红移,并带有附加的肩峰。为了了解该紫外吸收剂的紫外可见光谱变化,该紫外吸收谱溶解在有机溶剂中或作为纳米颗粒分散在水中,用不同介质中的MBBT分子的单体和聚集体进行密度泛函理论(DFT)计算。计算得出的分离的,即溶解的MBBT分子在乙醇和二恶烷中的UV-vis光谱与实验观察到的光谱非常吻合。在水分散体中观察到的实验UV-vis光谱的形状和位置变化不能仅凭溶剂效应来解释。发现所研究的分子可以形成稳定的,能量上有利的π堆积的二聚体,其显示的UV-vis光谱与在水分散体中实验观察到的光谱合理吻合。MBBT的此类聚集体很可能是在UV-vis吸收光谱中观察到红移的原因。此外,在二恶烷和水中使用时间依赖性DFT详细研究了激发的MBBT分子的光化学失活机理。通过MBBT在第一激发单重态下进行的分子内烯醇-酮互变异构作用在能量上最有利的途径是通过MBBT使吸收的能量失活。
更新日期:2020-01-17
中文翻译:

羟苯基-苯并三唑型紫外线吸收剂的光谱和光弛豫:从单体到纳米粒子。
水不溶性有机UV过滤剂,例如2,2'-亚甲基双-(6-(2H-苯并三唑-2-基)-4-(1,1,3,3-四甲基丁基)-苯酚)(MBBT)可以制备为纳米颗粒的水分散体。颗粒由各自的紫外线吸收剂分子组成,并表现出较强的紫外线吸收率。因为这种紫外线吸收剂在有机溶剂中具有一定的溶解度,所以也可以测量溶液在例如乙醇或二恶烷中的吸收光谱。水性分散体的紫外光谱显示出长波长带具有明显的红移,并带有附加的肩峰。为了了解该紫外吸收剂的紫外可见光谱变化,该紫外吸收谱溶解在有机溶剂中或作为纳米颗粒分散在水中,用不同介质中的MBBT分子的单体和聚集体进行密度泛函理论(DFT)计算。计算得出的分离的,即溶解的MBBT分子在乙醇和二恶烷中的UV-vis光谱与实验观察到的光谱非常吻合。在水分散体中观察到的实验UV-vis光谱的形状和位置变化不能仅凭溶剂效应来解释。发现所研究的分子可以形成稳定的,能量上有利的π堆积的二聚体,其显示的UV-vis光谱与在水分散体中实验观察到的光谱合理吻合。MBBT的此类聚集体很可能是在UV-vis吸收光谱中观察到红移的原因。此外,在二恶烷和水中使用时间依赖性DFT详细研究了激发的MBBT分子的光化学失活机理。通过MBBT在第一激发单重态下进行的分子内烯醇-酮互变异构作用在能量上最有利的途径是通过MBBT使吸收的能量失活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号