当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Integrated experimental and theoretical approach to probe the synergistic effect of ammonia in methanesulfonic acid reactions with small alkylamines.
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2020-01-06 , DOI: 10.1039/c9em00431a Véronique Perraud 1 , Jing Xu 2 , R Benny Gerber 3 , B J Finlayson-Pitts 1
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2020-01-06 , DOI: 10.1039/c9em00431a Véronique Perraud 1 , Jing Xu 2 , R Benny Gerber 3 , B J Finlayson-Pitts 1
Affiliation
|
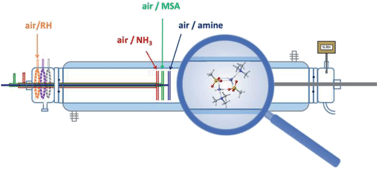
While new particle formation events have been observed worldwide, our fundamental understanding of the precursors remains uncertain. It has been previously shown that small alkylamines and ammonia (NH3) are key actors in sub-3 nm particle formation through reactions with acids such as sulfuric acid (H2SO4) and methanesulfonic acid (CH3S(O)(O)OH, MSA), and that water also plays a role. Because NH3 and amines co-exist in air, we carried out combined experimental and theoretical studies examining the influence of the addition of NH3 on particle formation from the reactions of MSA with methylamine (MA) and trimethylamine (TMA). Experiments were performed in a 1 m flow reactor at 1 atm and 296 K. Measurements using an ultrafine condensation particle counter (CPC) and a scanning mobility particle sizer (SMPS) show that new particle formation was systematically enhanced upon simultaneous addition of NH3 to the MSA + amine binary system, with the magnitude depending on the amine investigated. For the MSA + TMA reaction system, the addition of NH3 at ppb concentrations produced a much greater effect (i.e. order of magnitude more particles) than the addition of ∼12 000 ppm water (corresponding to ∼45-50% relative humidity). The effect of NH3 on the MSA + MA system, which is already very efficient in forming particles on its own, was present but modest. Calculations of energies, partial charges and structures of small cluster models of the multi-component particles likewise suggest synergistic effects due to NH3 in the presence of MSA and amine. The local minimum structures and the interactions involved suggest mechanisms for this effect.
中文翻译:

综合的实验和理论方法来探讨氨在甲烷磺酸与小烷基胺反应中的协同作用。
尽管在世界范围内已观察到新的颗粒形成事件,但我们对前体的基本了解仍不确定。先前已经证明,小烷基胺和氨(NH3)是通过与诸如硫酸(H2SO4)和甲磺酸(CH3S(O)(O)OH,MSA)之类的酸反应而形成亚3 nm颗粒的关键因素,而且水也起着作用。由于NH3和胺共存于空气中,因此我们进行了实验和理论研究相结合的研究,研究了NH3的添加对MSA与甲胺(MA)和三甲胺(TMA)反应形成颗粒的影响。实验是在1 m at 1 atm和296 K的1 m流动反应器中进行的。使用超细缩合颗粒计数器(CPC)和扫描迁移率粒度仪(SMPS)进行的测量表明,在将NH3同时添加到MSA +胺二元体系中后,新颗粒的形成得到系统地增强,其大小取决于所研究的胺。对于MSA + TMA反应系统,以ppb浓度添加NH3产生的效果(即增加颗粒数量级)要比添加〜12000 ppm的水(对应于〜45-50%相对湿度)产生更大的影响。NH3对MSA + MA系统的影响已经存在,但效果不明显,MSA + MA系统本身已经非常有效。多组分颗粒的小团簇模型的能量,部分电荷和结构的计算同样表明在MSA和胺存在下由于NH3而产生的协同效应。
更新日期:2020-02-26
中文翻译:

综合的实验和理论方法来探讨氨在甲烷磺酸与小烷基胺反应中的协同作用。
尽管在世界范围内已观察到新的颗粒形成事件,但我们对前体的基本了解仍不确定。先前已经证明,小烷基胺和氨(NH3)是通过与诸如硫酸(H2SO4)和甲磺酸(CH3S(O)(O)OH,MSA)之类的酸反应而形成亚3 nm颗粒的关键因素,而且水也起着作用。由于NH3和胺共存于空气中,因此我们进行了实验和理论研究相结合的研究,研究了NH3的添加对MSA与甲胺(MA)和三甲胺(TMA)反应形成颗粒的影响。实验是在1 m at 1 atm和296 K的1 m流动反应器中进行的。使用超细缩合颗粒计数器(CPC)和扫描迁移率粒度仪(SMPS)进行的测量表明,在将NH3同时添加到MSA +胺二元体系中后,新颗粒的形成得到系统地增强,其大小取决于所研究的胺。对于MSA + TMA反应系统,以ppb浓度添加NH3产生的效果(即增加颗粒数量级)要比添加〜12000 ppm的水(对应于〜45-50%相对湿度)产生更大的影响。NH3对MSA + MA系统的影响已经存在,但效果不明显,MSA + MA系统本身已经非常有效。多组分颗粒的小团簇模型的能量,部分电荷和结构的计算同样表明在MSA和胺存在下由于NH3而产生的协同效应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号