Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational Priming of RepA-WH1 for Functional Amyloid Conversion Detected by NMR Spectroscopy.
Structure ( IF 4.4 ) Pub Date : 2020-01-06 , DOI: 10.1016/j.str.2019.12.007 David Pantoja-Uceda 1 , Javier Oroz 1 , Cristina Fernández 2 , Eva de Alba 2 , Rafael Giraldo 2 , Douglas V Laurents 1
Structure ( IF 4.4 ) Pub Date : 2020-01-06 , DOI: 10.1016/j.str.2019.12.007 David Pantoja-Uceda 1 , Javier Oroz 1 , Cristina Fernández 2 , Eva de Alba 2 , Rafael Giraldo 2 , Douglas V Laurents 1
Affiliation
|
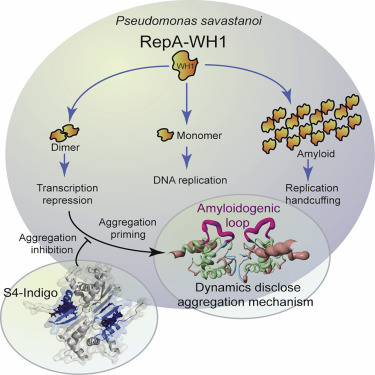
How proteins with a stable globular fold acquire the amyloid state is still largely unknown. RepA, a versatile plasmidic DNA binding protein from Pseudomonas savastanoi, is functional as a transcriptional repressor or as an initiator or inhibitor of DNA replication, the latter via assembly of an amyloidogenic oligomer. Its N-terminal domain (WH1) is responsible for discrimination between these functional abilities by undergoing insufficiently understood structural changes. RepA-WH1 is a stable dimer whose conformational dynamics had not been explored. Here, we have studied it through NMR {1H}-15N relaxation and H/D exchange kinetics measurements. The N- and the C-terminal α-helices, and the internal amyloidogenic loop, are partially unfolded in solution. S4-indigo, a small inhibitor of RepA-WH1 amyloidogenesis, binds to and tethers the N-terminal α-helix to a β-hairpin that is involved in dimerization, thus providing evidence for a priming role of fraying ends and dimerization switches in the amyloidogenesis of folded proteins.
中文翻译:

RepA-WH1的构象启动引物,用于通过核磁共振波谱法检测功能性淀粉样蛋白。
具有稳定的球状褶皱的蛋白质如何获得淀粉样状态仍然是未知的。RepA是一种来自Sseudomonas savastanoi的通用质粒DNA结合蛋白,可作为转录阻遏物或DNA复制的引发剂或抑制剂起作用,后者可通过产生淀粉样蛋白的低聚物进行组装。它的N末端结构域(WH1)负责通过不充分理解的结构变化来区分这些功能。RepA-WH1是一个稳定的二聚体,其构象动力学尚未探索。在这里,我们通过NMR {1H} -15N弛豫和H / D交换动力学测量研究了它。N和C端的α螺旋和内部淀粉样环在溶液中部分展开。S4-靛蓝,是RepA-WH1淀粉样蛋白生成的小抑制剂,
更新日期:2020-01-06
中文翻译:

RepA-WH1的构象启动引物,用于通过核磁共振波谱法检测功能性淀粉样蛋白。
具有稳定的球状褶皱的蛋白质如何获得淀粉样状态仍然是未知的。RepA是一种来自Sseudomonas savastanoi的通用质粒DNA结合蛋白,可作为转录阻遏物或DNA复制的引发剂或抑制剂起作用,后者可通过产生淀粉样蛋白的低聚物进行组装。它的N末端结构域(WH1)负责通过不充分理解的结构变化来区分这些功能。RepA-WH1是一个稳定的二聚体,其构象动力学尚未探索。在这里,我们通过NMR {1H} -15N弛豫和H / D交换动力学测量研究了它。N和C端的α螺旋和内部淀粉样环在溶液中部分展开。S4-靛蓝,是RepA-WH1淀粉样蛋白生成的小抑制剂,











































 京公网安备 11010802027423号
京公网安备 11010802027423号