Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
USP15 potentiates NF-κB activation by differentially stabilizing TAB2 and TAB3.
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-01-05 , DOI: 10.1111/febs.15202 Qiaoqiao Zhou 1 , Cheng Cheng 1 , Yujuan Wei 1 , Jing Yang 1 , Wanzhu Zhou 1 , Qiuyi Song 1 , Mengxiang Ke 1 , Wanyao Yan 1 , Ling Zheng 2 , Yu Zhang 1 , Kun Huang 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-01-05 , DOI: 10.1111/febs.15202 Qiaoqiao Zhou 1 , Cheng Cheng 1 , Yujuan Wei 1 , Jing Yang 1 , Wanzhu Zhou 1 , Qiuyi Song 1 , Mengxiang Ke 1 , Wanyao Yan 1 , Ling Zheng 2 , Yu Zhang 1 , Kun Huang 1
Affiliation
|
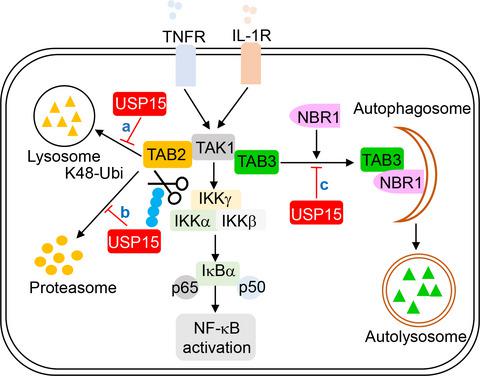
Tumor necrosis factor α (TNFα)‐ and interleukin 1β (IL‐1β)‐induced nuclear factor‐κB (NF‐κB) activation play key roles in inflammation, immunity, and cancer development. Here, we identified one of the deubiquitinating enzymes (DUBs), ubiquitin‐specific protease 15 (USP15), as a positive regulator in both TNFα‐ and IL‐1β‐induced NF‐κB activation. Overexpression of USP15 potentiated TNFα‐ or IL‐1β‐triggered NF‐κB activation and downstream gene transcription, whereas knockdown of USP15 had opposite effects. Mechanistically, upon TNFα stimulation, USP15 showed an enhanced interaction with transforming growth factor‐β activated kinase‐1 (TAK1)‐TAK1 binding protein (TAB) complex to inhibit the proteolysis of TAB2/3 by different pathways. Apart from deubiquitination dependently inducing cleavage of lysine 48‐linked TAB2 ubiquitination, USP15 also DUB independently inhibited lysosome‐associated TAB2 degradation, thus enhanced TAB2 stabilization. For TAB3, USP15 inhibited NBR1‐mediated selective autophagic TAB3 degradation independent of its deubiquitinating activity. Together, our results reveal a novel USP15‐mediated mechanism through which efficient NF‐κB activation is achieved by differentially maintaining the TAB2/3 stability.
中文翻译:

USP15通过差异稳定TAB2和TAB3来增强NF-κB的激活。
肿瘤坏死因子α(TNFα)和白介素1β(IL-1β)诱导的核因子κB(NF-κB)激活在炎症,免疫力和癌症发展中起关键作用。在这里,我们确定了一种去泛素化酶(DUBs),即泛素特异性蛋白酶15(USP15),是TNFα和IL-1β诱导的NF-κB激活的正调节剂。USP15的过表达增强了TNFα或IL-1β触发的NF-κB激活和下游基因转录,而敲低USP15具有相反的作用。从机制上讲,在TNFα刺激下,USP15与转化生长因子-β活化激酶-1(TAK1)-TAK1结合蛋白(TAB)复合物之间的相互作用增强,从而可以通过不同途径抑制TAB2 / 3的蛋白水解。除了去泛素化可诱导赖氨酸48连锁的TAB2泛素化裂解外,USP15还是DUB独立地抑制了溶酶体相关的TAB2降解,从而增强了TAB2的稳定性。对于TAB3,USP15抑制NBR1介导的选择性自噬TAB3降解,而与其去泛素化活性无关。总之,我们的结果揭示了一种新型的USP15介导的机制,通过该机制可以通过差异性地维持TAB2 / 3的稳定性来实现有效的NF-κB活化。
更新日期:2020-01-05
中文翻译:

USP15通过差异稳定TAB2和TAB3来增强NF-κB的激活。
肿瘤坏死因子α(TNFα)和白介素1β(IL-1β)诱导的核因子κB(NF-κB)激活在炎症,免疫力和癌症发展中起关键作用。在这里,我们确定了一种去泛素化酶(DUBs),即泛素特异性蛋白酶15(USP15),是TNFα和IL-1β诱导的NF-κB激活的正调节剂。USP15的过表达增强了TNFα或IL-1β触发的NF-κB激活和下游基因转录,而敲低USP15具有相反的作用。从机制上讲,在TNFα刺激下,USP15与转化生长因子-β活化激酶-1(TAK1)-TAK1结合蛋白(TAB)复合物之间的相互作用增强,从而可以通过不同途径抑制TAB2 / 3的蛋白水解。除了去泛素化可诱导赖氨酸48连锁的TAB2泛素化裂解外,USP15还是DUB独立地抑制了溶酶体相关的TAB2降解,从而增强了TAB2的稳定性。对于TAB3,USP15抑制NBR1介导的选择性自噬TAB3降解,而与其去泛素化活性无关。总之,我们的结果揭示了一种新型的USP15介导的机制,通过该机制可以通过差异性地维持TAB2 / 3的稳定性来实现有效的NF-κB活化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号