当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Isoquinoline Derivatives via Palladium‐Catalyzed C−H/C−N Bond Activation of N‐Acyl Hydrazones with α‐Substituted Vinyl Azides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-02-14 , DOI: 10.1002/adsc.201901394 Biao Nie 1 , Wanqing Wu 1 , Wei Zeng 1 , Qingyun Ren 2 , Ji Zhang 2 , Yingjun Zhang 2 , Huanfeng Jiang 1, 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-02-14 , DOI: 10.1002/adsc.201901394 Biao Nie 1 , Wanqing Wu 1 , Wei Zeng 1 , Qingyun Ren 2 , Ji Zhang 2 , Yingjun Zhang 2 , Huanfeng Jiang 1, 3
Affiliation
|
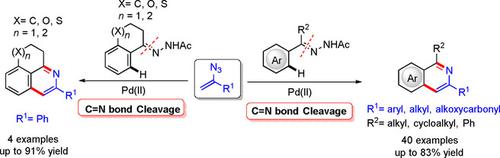
A palladium‐catalyzed cyclization of N‐acetyl hydrazones with vinyl azides has been developed. Various substituted isoquinolines, including diverse fused isoquinolines can be prepared via this protocol in moderate to good yields. Mechanistic studies suggest that α‐substituted vinyl azide serves as an internal nitrogen source. Also, C−H bond activation and C−N bond cleavage have been realized using hydrazone as directing group.
中文翻译:

通过钯催化的α-取代的乙烯基叠氮化物对N-酰基Hy的C-H / C-N键活化,合成异喹啉衍生物
已经开发了钯催化的乙烯基叠氮化物对N乙酰基hydr的环化反应。可以通过该方案以中等至良好的产率制备各种取代的异喹啉,包括各种稠合的异喹啉。机理研究表明,α-取代的叠氮化乙烯可作为内部氮源。另外,使用作为指导基团已经实现了CH键的活化和CN键的裂解。
更新日期:2020-02-14
中文翻译:

通过钯催化的α-取代的乙烯基叠氮化物对N-酰基Hy的C-H / C-N键活化,合成异喹啉衍生物
已经开发了钯催化的乙烯基叠氮化物对N乙酰基hydr的环化反应。可以通过该方案以中等至良好的产率制备各种取代的异喹啉,包括各种稠合的异喹啉。机理研究表明,α-取代的叠氮化乙烯可作为内部氮源。另外,使用作为指导基团已经实现了CH键的活化和CN键的裂解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号