当前位置:
X-MOL 学术
›
Cancer Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of folate receptor α (FRα) binding oligopeptides and their evaluation for targeted virotherapy applications.
Cancer Gene Therapy ( IF 4.8 ) Pub Date : 2020-01-06 , DOI: 10.1038/s41417-019-0156-0 Sarah L Hulin-Curtis 1 , James A Davies 1 , Davor Nestić 2 , Emily A Bates 1 , Alexander T Baker 1 , Tabitha G Cunliffe 1 , Dragomira Majhen 2 , John D Chester 1, 3 , Alan L Parker 1
Cancer Gene Therapy ( IF 4.8 ) Pub Date : 2020-01-06 , DOI: 10.1038/s41417-019-0156-0 Sarah L Hulin-Curtis 1 , James A Davies 1 , Davor Nestić 2 , Emily A Bates 1 , Alexander T Baker 1 , Tabitha G Cunliffe 1 , Dragomira Majhen 2 , John D Chester 1, 3 , Alan L Parker 1
Affiliation
|
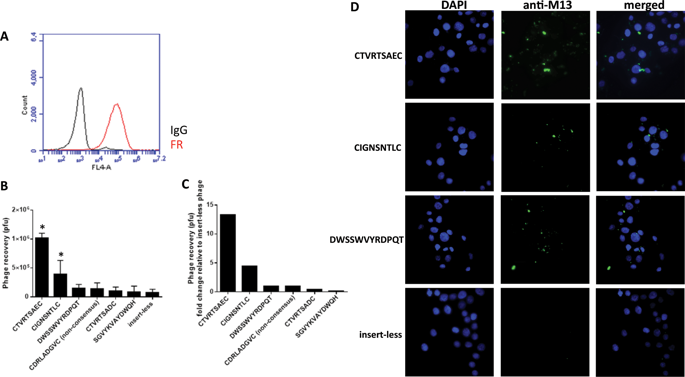
Oncolytic virotherapies (OV) based on human adenoviral (HAdV) vectors hold significant promise for the treatment of advanced ovarian cancers where local, intraperitoneal delivery to tumour metastases is feasible, bypassing many complexities associated with intravascular delivery. The efficacy of HAdV-C5-based OV is hampered by a lack of tumour selectivity, where the primary receptor, hCAR, is commonly downregulated during malignant transformation. Conversely, folate receptor alpha (FRα) is highly expressed on ovarian cancer cells, providing a compelling target for tumour selective delivery of virotherapies. Here, we identify high-affinity FRα-binding oligopeptides for genetic incorporation into HAdV-C5 vectors. Biopanning identified a 12-mer linear peptide, DWSSWVYRDPQT, and two 7-mer cysteine-constrained peptides, CIGNSNTLC and CTVRTSAEC that bound FRα in the context of the phage particle. Synthesised lead peptide, CTVRTSAEC, bound specifically to FRα and could be competitively inhibited with folic acid. To assess the capacity of the elucidated FRα-binding oligopeptides to target OV to FRα, we genetically incorporated the peptides into the HAdV-C5 fiber-knob HI loop including in vectors genetically ablated for hCAR interactions. Unfortunately, the recombinant vectors failed to efficiently target transduction via FRα due to defective intracellular trafficking following entry via FRα, indicating that whilst the peptides identified may have potential for applications for targeted drug delivery, they require additional refinement for targeted virotherapy applications.
中文翻译:

叶酸受体 α (FRα) 结合寡肽的鉴定及其针对靶向病毒治疗应用的评估。
基于人腺病毒(HAdV)载体的溶瘤病毒疗法(OV)对于治疗晚期卵巢癌具有重大前景,其中局部腹膜内递送至肿瘤转移是可行的,绕过了与血管内递送相关的许多复杂性。基于 HAdV-C5 的 OV 的功效因缺乏肿瘤选择性而受到阻碍,其中主要受体 hCAR 在恶性转化过程中通常会下调。相反,叶酸受体α(FRα)在卵巢癌细胞上高表达,为肿瘤选择性递送病毒疗法提供了引人注目的靶点。在这里,我们鉴定了高亲和力 FRα 结合寡肽,用于基因整合到 HAdV-C5 载体中。生物淘选鉴定出一个 12 聚体线性肽 DWSSWVYRDPQT 和两个 7 聚体半胱氨酸限制肽 CIGNSNTLC 和 CTVRTSAEC,它们在噬菌体颗粒的背景下结合 FRα。合成的先导肽 CTVRTSAEC 与 FRα 特异性结合,并且可以被叶酸竞争性抑制。为了评估已阐明的 FRα 结合寡肽将 OV 靶向 FRα 的能力,我们将这些肽基因整合到 HAdV-C5 纤维旋钮 HI 环中,包括基因消融用于 hCAR 相互作用的载体中。不幸的是,由于通过 FRα 进入后细胞内运输存在缺陷,重组载体未能有效地通过 FRα 进行靶向转导,这表明虽然所鉴定的肽可能具有用于靶向药物递送的应用潜力,但它们需要针对靶向病毒治疗应用进行额外的改进。
更新日期:2020-01-06
中文翻译:

叶酸受体 α (FRα) 结合寡肽的鉴定及其针对靶向病毒治疗应用的评估。
基于人腺病毒(HAdV)载体的溶瘤病毒疗法(OV)对于治疗晚期卵巢癌具有重大前景,其中局部腹膜内递送至肿瘤转移是可行的,绕过了与血管内递送相关的许多复杂性。基于 HAdV-C5 的 OV 的功效因缺乏肿瘤选择性而受到阻碍,其中主要受体 hCAR 在恶性转化过程中通常会下调。相反,叶酸受体α(FRα)在卵巢癌细胞上高表达,为肿瘤选择性递送病毒疗法提供了引人注目的靶点。在这里,我们鉴定了高亲和力 FRα 结合寡肽,用于基因整合到 HAdV-C5 载体中。生物淘选鉴定出一个 12 聚体线性肽 DWSSWVYRDPQT 和两个 7 聚体半胱氨酸限制肽 CIGNSNTLC 和 CTVRTSAEC,它们在噬菌体颗粒的背景下结合 FRα。合成的先导肽 CTVRTSAEC 与 FRα 特异性结合,并且可以被叶酸竞争性抑制。为了评估已阐明的 FRα 结合寡肽将 OV 靶向 FRα 的能力,我们将这些肽基因整合到 HAdV-C5 纤维旋钮 HI 环中,包括基因消融用于 hCAR 相互作用的载体中。不幸的是,由于通过 FRα 进入后细胞内运输存在缺陷,重组载体未能有效地通过 FRα 进行靶向转导,这表明虽然所鉴定的肽可能具有用于靶向药物递送的应用潜力,但它们需要针对靶向病毒治疗应用进行额外的改进。











































 京公网安备 11010802027423号
京公网安备 11010802027423号