当前位置:
X-MOL 学术
›
Genet. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficacy and safety of D,L-3-hydroxybutyrate (D,L-3-HB) treatment in multiple acyl-CoA dehydrogenase deficiency.
Genetics in Medicine ( IF 6.6 ) Pub Date : 2020-01-06 , DOI: 10.1038/s41436-019-0739-z Willemijn J van Rijt 1 , Emmalie A Jager 1 , Derk P Allersma 2 , A Çiğdem Aktuğlu Zeybek 3 , Kaustuv Bhattacharya 4 , François-Guillaume Debray 5 , Carolyn J Ellaway 4 , Matthias Gautschi 6 , Michael T Geraghty 7 , David Gil-Ortega 8 , Austin A Larson 9 , Francesca Moore 10 , Eva Morava 11, 12 , Andrew A Morris 13, 14 , Kimihiko Oishi 15 , Manuel Schiff 16 , Sabine Scholl-Bürgi 17 , Michel C Tchan 18 , Jerry Vockley 19 , Peter Witters 12 , Saskia B Wortmann 20, 21, 22 , Francjan van Spronsen 1 , Johan L K Van Hove 9 , Terry G J Derks 1
Genetics in Medicine ( IF 6.6 ) Pub Date : 2020-01-06 , DOI: 10.1038/s41436-019-0739-z Willemijn J van Rijt 1 , Emmalie A Jager 1 , Derk P Allersma 2 , A Çiğdem Aktuğlu Zeybek 3 , Kaustuv Bhattacharya 4 , François-Guillaume Debray 5 , Carolyn J Ellaway 4 , Matthias Gautschi 6 , Michael T Geraghty 7 , David Gil-Ortega 8 , Austin A Larson 9 , Francesca Moore 10 , Eva Morava 11, 12 , Andrew A Morris 13, 14 , Kimihiko Oishi 15 , Manuel Schiff 16 , Sabine Scholl-Bürgi 17 , Michel C Tchan 18 , Jerry Vockley 19 , Peter Witters 12 , Saskia B Wortmann 20, 21, 22 , Francjan van Spronsen 1 , Johan L K Van Hove 9 , Terry G J Derks 1
Affiliation
|
PURPOSE
Multiple acyl-CoA dehydrogenase deficiency (MADD) is a life-threatening, ultrarare inborn error of metabolism. Case reports described successful D,L-3-hydroxybutyrate (D,L-3-HB) treatment in severely affected MADD patients, but systematic data on efficacy and safety is lacking.
METHODS
A systematic literature review and an international, retrospective cohort study on clinical presentation, D,L-3-HB treatment method, and outcome in MADD(-like) patients.
RESULTS
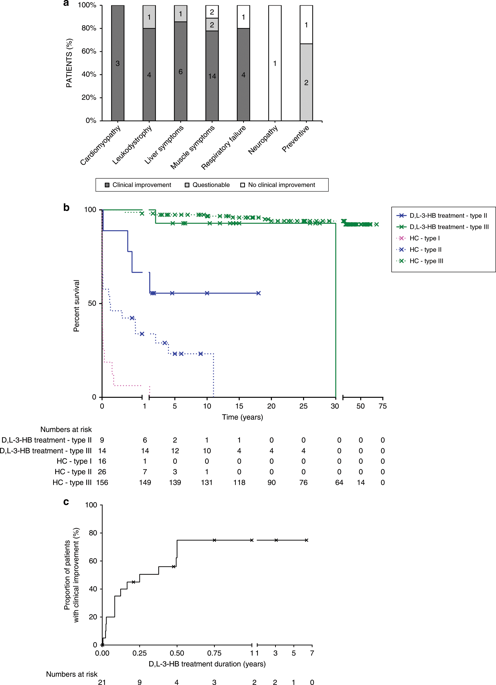
Our study summarizes 23 MADD(-like) patients, including 14 new cases. Median age at clinical onset was two months (interquartile range [IQR]: 8 months). Median age at starting D,L-3-HB was seven months (IQR: 4.5 years). D,L-3-HB doses ranged between 100 and 2600 mg/kg/day. Clinical improvement was reported in 16 patients (70%) for cardiomyopathy, leukodystrophy, liver symptoms, muscle symptoms, and/or respiratory failure. D,L-3-HB appeared not effective for neuropathy. Survival appeared longer upon D,L-3-HB compared with historical controls. Median time until first clinical improvement was one month, and ranged up to six months. Reported side effects included abdominal pain, constipation, dehydration, diarrhea, and vomiting/nausea. Median D,L-3-HB treatment duration was two years (IQR: 6 years). D,L-3-HB treatment was discontinued in 12 patients (52%).
CONCLUSION
The strength of the current study is the international pooling of data demonstrating that D,L-3-HB treatment can be effective and safe in MADD(-like) patients.
中文翻译:

D,L-3-羟基丁酸 (D,L-3-HB) 治疗多种酰基辅酶 A 脱氢酶缺乏症的疗效和安全性。
目的 多酰基辅酶 A 脱氢酶缺乏症 (MADD) 是一种危及生命的极其罕见的先天性代谢缺陷。病例报告描述了 D,L-3-羟基丁酸 (D,L-3-HB) 对严重受影响的 MADD 患者的成功治疗,但缺乏关于疗效和安全性的系统数据。方法 对 MADD 类患者的临床表现、D,L-3-HB 治疗方法和结局进行系统文献回顾和国际回顾性队列研究。结果我们的研究总结了 23 例 MADD(类似)患者,其中包括 14 例新病例。临床发病时的中位年龄为两个月(四分位数范围 [IQR]:8 个月)。开始 D,L-3-HB 的中位年龄为 7 个月(IQR:4.5 岁)。 D,L-3-HB 剂量范围为 100 至 2600 mg/kg/天。据报道,16 名患者 (70%) 的心肌病、脑白质营养不良、肝脏症状、肌肉症状和/或呼吸衰竭的临床改善。 D,L-3-HB 似乎对神经病变无效。与历史对照相比,D,L-3-HB 的生存期似乎更长。第一次临床改善的中位时间为一个月,最长可达六个月。报告的副作用包括腹痛、便秘、脱水、腹泻和呕吐/恶心。中位 D,L-3-HB 治疗持续时间为两年(IQR:6 年)。 12 名患者 (52%) 停止 D,L-3-HB 治疗。结论 本研究的优势在于国际数据汇集证明 D,L-3-HB 治疗对于 MADD(类似)患者是有效且安全的。
更新日期:2020-01-06
中文翻译:

D,L-3-羟基丁酸 (D,L-3-HB) 治疗多种酰基辅酶 A 脱氢酶缺乏症的疗效和安全性。
目的 多酰基辅酶 A 脱氢酶缺乏症 (MADD) 是一种危及生命的极其罕见的先天性代谢缺陷。病例报告描述了 D,L-3-羟基丁酸 (D,L-3-HB) 对严重受影响的 MADD 患者的成功治疗,但缺乏关于疗效和安全性的系统数据。方法 对 MADD 类患者的临床表现、D,L-3-HB 治疗方法和结局进行系统文献回顾和国际回顾性队列研究。结果我们的研究总结了 23 例 MADD(类似)患者,其中包括 14 例新病例。临床发病时的中位年龄为两个月(四分位数范围 [IQR]:8 个月)。开始 D,L-3-HB 的中位年龄为 7 个月(IQR:4.5 岁)。 D,L-3-HB 剂量范围为 100 至 2600 mg/kg/天。据报道,16 名患者 (70%) 的心肌病、脑白质营养不良、肝脏症状、肌肉症状和/或呼吸衰竭的临床改善。 D,L-3-HB 似乎对神经病变无效。与历史对照相比,D,L-3-HB 的生存期似乎更长。第一次临床改善的中位时间为一个月,最长可达六个月。报告的副作用包括腹痛、便秘、脱水、腹泻和呕吐/恶心。中位 D,L-3-HB 治疗持续时间为两年(IQR:6 年)。 12 名患者 (52%) 停止 D,L-3-HB 治疗。结论 本研究的优势在于国际数据汇集证明 D,L-3-HB 治疗对于 MADD(类似)患者是有效且安全的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号