当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective (3+2) cycloaddition via N-heterocyclic carbene-catalyzed addition of homoenolates to cyclic N-sulfonyl trifluoromethylated ketimines: synthesis of fused N-heterocycle γ-lactams.
Chemical Communications ( IF 4.3 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9cc09269b Zhen-Zhen Zhang 1 , Yongna Zhang , Hui-Xin Duan , Zhuo-Fei Deng , You-Qing Wang
Chemical Communications ( IF 4.3 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9cc09269b Zhen-Zhen Zhang 1 , Yongna Zhang , Hui-Xin Duan , Zhuo-Fei Deng , You-Qing Wang
Affiliation
|
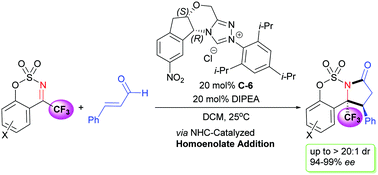
An enantioselective (3+2) cycloaddition of enals and cyclic N-sulfonyl trifluoromethyl ketimines via N-heterocyclic carbene-catalyzed homoenolate addition is described. This reaction can efficiently construct fused N-heterocycle γ-lactams bearing two adjacent chiral centers with >20 : 1 dr and 94-99% ee, with one chiral center as a trifluoromethylated α-tetrasubstituted carbon stereocenter.
中文翻译:

通过将N-杂环卡宾催化的均烯酸酯加成到环状N-磺酰基三氟甲基化酮亚胺上的对映选择性(3 + 2)环加成反应:稠合的N杂环γ-内酰胺的合成。
描述了通过N-杂环卡宾催化的均烯酸酯加成对映体和环状N-磺酰基三氟甲基酮亚胺的对映选择性(3 + 2)环加成。该反应可以有效地构建稠合的N-杂环γ-内酰胺,其带有两个相邻的手性中心,具有> 20:1 dr和94-99%ee,一个手性中心为三氟甲基化的α-四取代碳立体中心。
更新日期:2020-01-13
中文翻译:

通过将N-杂环卡宾催化的均烯酸酯加成到环状N-磺酰基三氟甲基化酮亚胺上的对映选择性(3 + 2)环加成反应:稠合的N杂环γ-内酰胺的合成。
描述了通过N-杂环卡宾催化的均烯酸酯加成对映体和环状N-磺酰基三氟甲基酮亚胺的对映选择性(3 + 2)环加成。该反应可以有效地构建稠合的N-杂环γ-内酰胺,其带有两个相邻的手性中心,具有> 20:1 dr和94-99%ee,一个手性中心为三氟甲基化的α-四取代碳立体中心。











































 京公网安备 11010802027423号
京公网安备 11010802027423号