Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of exo-Spiro[2′,2′-difluorocyclopropane-3′,2′-tropanes]
Synthesis ( IF 2.2 ) Pub Date : 2020-01-02 , DOI: 10.1055/s-0039-1691560 Andrii Gerasov 1 , Grygoriy A. Dolgonos 2 , Aleksandr Yu. Mandzhulo 1, 3 , Alexey Ryabitsky 1 , Volodymyr Fetyukhin 1 , Oleg Lukin 1 , Alexander Shivanyuk 1, 4
Synthesis ( IF 2.2 ) Pub Date : 2020-01-02 , DOI: 10.1055/s-0039-1691560 Andrii Gerasov 1 , Grygoriy A. Dolgonos 2 , Aleksandr Yu. Mandzhulo 1, 3 , Alexey Ryabitsky 1 , Volodymyr Fetyukhin 1 , Oleg Lukin 1 , Alexander Shivanyuk 1, 4
Affiliation
|
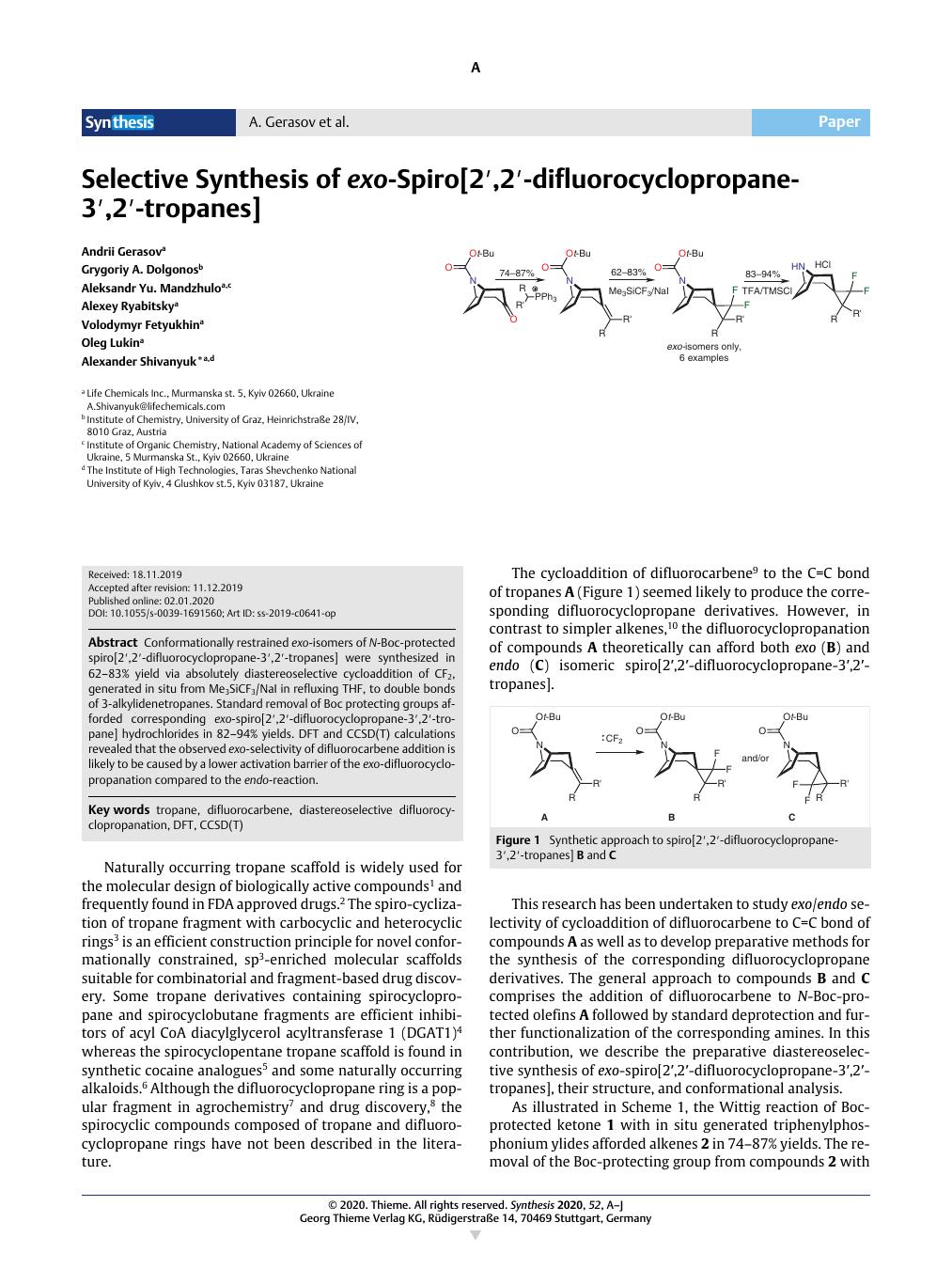
Conformationally restrained exo-isomers of N-Boc-protected spiro[2′,2′-difluorocyclopropane-3′,2′-tropanes] were synthesized in 62–83% yield via absolutely diastereoselective cycloaddition of CF2, generated in situ from Me3SiCF3/NaI in refluxing THF, to double bonds of 3-alkylidenetropanes. Standard removal of Boc protecting groups afforded corresponding exo-spiro[2′,2′-difluorocyclopropane-3′,2′-tropane] hydrochlorides in 82–94% yields. DFT and CCSD(T) calculations revealed that the observed exo-selectivity of difluorocarbene addition is likely to be caused by a lower activation barrier of the exo-difluorocyclopropanation compared to the endo-reaction.
中文翻译:

exo-Spiro [2',2'-二氟环丙烷-3',2'-托烷的选择性合成
构象限制外的异构体Ñ -Boc保护的螺[2',2'-二氟环丙烷-3',2'-莨菪烷]通过CF的非对映选择性绝对环加成在62-83%的产率合成了2,选自Me原位产生的3 SiCF 3 / NaI在回流的THF中形成3-烷基亚烷基戊烷的双键。标准去除Boc保护基团可提供82-94%的产率的相应的exo- spiro [2',2'-difluorocyclopropane-3',2'-tropane]盐酸盐。DFT和CCSD(T)的计算表明,所观察到的外型二氟卡宾的加成是-选择性可能受到的下活化屏障引起的外-二氟环丙烷化与内-反应相比。
更新日期:2020-01-04
中文翻译:

exo-Spiro [2',2'-二氟环丙烷-3',2'-托烷的选择性合成
构象限制外的异构体Ñ -Boc保护的螺[2',2'-二氟环丙烷-3',2'-莨菪烷]通过CF的非对映选择性绝对环加成在62-83%的产率合成了2,选自Me原位产生的3 SiCF 3 / NaI在回流的THF中形成3-烷基亚烷基戊烷的双键。标准去除Boc保护基团可提供82-94%的产率的相应的exo- spiro [2',2'-difluorocyclopropane-3',2'-tropane]盐酸盐。DFT和CCSD(T)的计算表明,所观察到的外型二氟卡宾的加成是-选择性可能受到的下活化屏障引起的外-二氟环丙烷化与内-反应相比。











































 京公网安备 11010802027423号
京公网安备 11010802027423号