当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-locking stabilization of covalent helical peptides: Application to Bfl-1 antagonists.
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-01-20 , DOI: 10.1111/cbdd.13661 Carlo Baggio 1 , Parima Udompholkul 1 , Luca Gambini 1 , Jennifer Jossart 2 , Ahmed F Salem 1 , Maria Håkansson 3 , J Jefferson P Perry 2 , Maurizio Pellecchia 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-01-20 , DOI: 10.1111/cbdd.13661 Carlo Baggio 1 , Parima Udompholkul 1 , Luca Gambini 1 , Jennifer Jossart 2 , Ahmed F Salem 1 , Maria Håkansson 3 , J Jefferson P Perry 2 , Maurizio Pellecchia 1
Affiliation
|
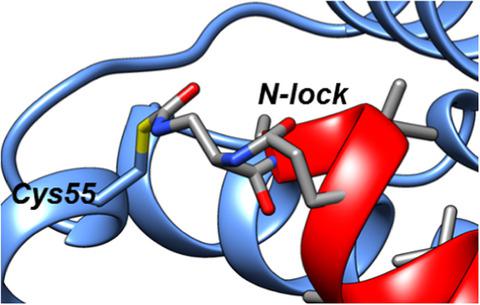
Recently, it was reported that tetrapeptides cyclized via lactam bond between the amino terminus and a glutamic residue in position 4 (termed here N-lock) can nucleate helix formation in longer peptides. We applied such strategy to derive N-locked covalent BH3 peptides that were designed to selectively target the anti-apoptotic protein Bfl-1. The resulting agents were soluble in aqueous buffer and displayed a remarkable (low nanomolar) affinity for Bfl-1 and cellular activity. The crystal structure of the complex between such N-locked covalent peptide and Bfl-1 provided insights on the geometry of the N-locking strategy and of the covalent bond between the agent and Bfl-1.
中文翻译:

共价螺旋肽的N锁稳定作用:应用于Bfl-1拮抗剂。
最近,据报道,通过氨基末端和位置4的谷氨酸残基之间的内酰胺键环化的四肽(在此称为N-锁)可以使更长的肽中的螺旋形成成核。我们应用这种策略来衍生N锁的共价BH3肽,旨在选择性地靶向抗凋亡蛋白Bfl-1。所得试剂可溶于水性缓冲液,并显示出对Bfl-1和细胞活性的显着(低纳摩尔)亲和力。这种N-锁定的共价肽和Bfl-1之间的复合物的晶体结构提供了关于N-锁定策略的几何形状以及试剂与Bfl-1之间的共价键的见解。
更新日期:2020-01-20
中文翻译:

共价螺旋肽的N锁稳定作用:应用于Bfl-1拮抗剂。
最近,据报道,通过氨基末端和位置4的谷氨酸残基之间的内酰胺键环化的四肽(在此称为N-锁)可以使更长的肽中的螺旋形成成核。我们应用这种策略来衍生N锁的共价BH3肽,旨在选择性地靶向抗凋亡蛋白Bfl-1。所得试剂可溶于水性缓冲液,并显示出对Bfl-1和细胞活性的显着(低纳摩尔)亲和力。这种N-锁定的共价肽和Bfl-1之间的复合物的晶体结构提供了关于N-锁定策略的几何形状以及试剂与Bfl-1之间的共价键的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号