当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electroactivated alkylation of amines with alcohols via both direct and indirect borrowing hydrogen mechanisms
Green Chemistry ( IF 9.3 ) Pub Date : 2020/01/03 , DOI: 10.1039/c9gc03747k Benjamin Appiagyei 1, 2, 3, 4 , Souful Bhatia 1, 2, 3, 4 , Gabriela L. Keeney 1, 2, 3, 4 , Troy Dolmetsch 1, 2, 3, 4 , James E. Jackson 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2020/01/03 , DOI: 10.1039/c9gc03747k Benjamin Appiagyei 1, 2, 3, 4 , Souful Bhatia 1, 2, 3, 4 , Gabriela L. Keeney 1, 2, 3, 4 , Troy Dolmetsch 1, 2, 3, 4 , James E. Jackson 1, 2, 3, 4
Affiliation
|
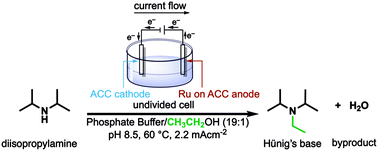
A green, efficient N-alkylation of amines with simple alcohols has been achieved in aqueous solution via an electrochemical version of the so-called “borrowing hydrogen methodology”. Catalyzed by Ru on activated carbon cloth (Ru/ACC), the reaction works well with methanol, and with primary and secondary alcohols. Alkylation can be accomplished by either of two different electrocatalytic processes: (1) in an undivided cell, alcohol (present in excess) is oxidized at the Ru/ACC anode; the aldehyde or ketone product condenses with the amine; and the resulting imine is reduced at an ACC cathode, combining with protons released by the oxidation. This process consumes stoichiometric quantities of current. (2) In a membrane-divided cell, the current-activated Ru/ACC cathode effects direct C–H activation of the alcohol; the resulting carbonyl species, either free or still surface-adsorbed, condenses with amine to form imine and is reduced as in (1). These alcohol activation processes can alkylate primary and secondary aliphatic amines, as well as ammonia itself at 25–70 °C and ambient pressure.
中文翻译:

通过直接和间接借位氢机理将胺与醇进行电活化烷基化
在水溶液中,通过简单的醇可实现胺的绿色高效N-烷基化所谓的“借用氢方法”的电化学形式。在活性炭布(Ru / ACC)上,通过Ru催化,该反应在甲醇,伯醇和仲醇中均能很好地进行。烷基化可以通过两种不同的电催化方法之一完成:(1)在未分隔的电池中,醇(过量存在)在Ru / ACC阳极被氧化;醛或酮产物与胺缩合;并在ACC阴极还原生成的亚胺,并与氧化释放的质子结合。该过程消耗化学计量的电流。(2)在膜分裂的电池中,电流激活的Ru / ACC阴极直接影响乙醇的C–H激活;所得的游离或仍表面吸附的羰基物质与胺缩合形成亚胺,并按(1)所述还原。
更新日期:2020-02-13
中文翻译:

通过直接和间接借位氢机理将胺与醇进行电活化烷基化
在水溶液中,通过简单的醇可实现胺的绿色高效N-烷基化所谓的“借用氢方法”的电化学形式。在活性炭布(Ru / ACC)上,通过Ru催化,该反应在甲醇,伯醇和仲醇中均能很好地进行。烷基化可以通过两种不同的电催化方法之一完成:(1)在未分隔的电池中,醇(过量存在)在Ru / ACC阳极被氧化;醛或酮产物与胺缩合;并在ACC阴极还原生成的亚胺,并与氧化释放的质子结合。该过程消耗化学计量的电流。(2)在膜分裂的电池中,电流激活的Ru / ACC阴极直接影响乙醇的C–H激活;所得的游离或仍表面吸附的羰基物质与胺缩合形成亚胺,并按(1)所述还原。











































 京公网安备 11010802027423号
京公网安备 11010802027423号