当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How coverage influences thermodynamic and kinetic isotope effects for H2/D2 dissociative adsorption on transition metals
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2019/12/10 , DOI: 10.1039/c9cy02338k Benjamin W. J. Chen 1, 2, 3, 4 , Manos Mavrikakis 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2019/12/10 , DOI: 10.1039/c9cy02338k Benjamin W. J. Chen 1, 2, 3, 4 , Manos Mavrikakis 1, 2, 3, 4
Affiliation
|
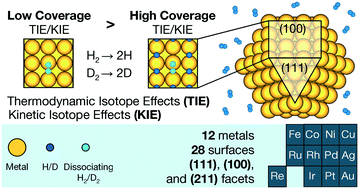
Isotope effects greatly enhance our understanding of chemical reactions in heterogeneous catalysis. Despite their widespread use, there is limited understanding about how realistic reaction conditions, such as the coverage of surface adsorbates, affect them. Here, we study the influence of hydrogen (H) coverage on the thermodynamic and kinetic isotope effects of H2/D2 dissociative adsorption on the close-packed, open, and stepped surfaces of 12 transition metals: Ag, Au, Co, Cu, Fe, Ir, Ni, Re, Pd, Pt, Rh, and Ru, over a catalytically relevant temperature range. Through first-principles density functional theory calculations, we show that increasing coverage has two effects: i) it may change preferred adsorption sites and transition state geometries, and ii) it increases the vibrational frequencies of adsorbed H due to the interactions between H atoms. Empirically, isotope effects decrease in absolute value with increasing coverage for most of our studied systems, indicating a relative shift in stability in favor of the D-substituted minima and transition states. This is likely due to the consistent influence of the latter factor, which affects all structures. Higher temperatures reduce the magnitude of these decreases. Our findings provide insights into the nanoscale mechanisms by which coverage influences isotope effects, which will affect how we interpret experimentally measured isotope effects. They also point towards new applications of isotope effects in catalysis, such as for quantifying adsorbate coverages as well as for elucidating adsorption and active sites on the surfaces of catalysts.
中文翻译:

覆盖率如何影响过渡金属上H2 / D2的解离吸附的热力学和动力学同位素效应
同位素效应极大地增进了我们对非均相催化中化学反应的理解。尽管已广泛使用它们,但对于现实的反应条件(例如表面被吸附物的覆盖范围)如何影响它们的理解仍然有限。在这里,我们研究氢(H)的覆盖对H 2 / D 2的热力学和动力学同位素效应的影响在催化相关的温度范围内,在12种过渡金属:Ag,Au,Co,Cu,Fe,Ir,Ni,Re,Pd,Pt,Rh和Ru的密排,开放和阶梯状表面上发生解离吸附。通过第一原理密度泛函理论计算,我们发现增加覆盖率具有两个作用:i)可能会改变首选的吸附位点和过渡态的几何形状,ii)由于H原子之间的相互作用而增加了被吸附H的振动频率。根据经验,对于大多数我们研究的系统,同位素效应的绝对值随覆盖率的增加而减小,表明稳定性的相对变化有利于D取代的最小值和过渡态。这可能是由于后一因素的持续影响,后者影响了所有结构。较高的温度降低了这些降低的幅度。我们的发现为覆盖机制影响同位素效应的纳米尺度机理提供了见识,而纳米机制将影响我们解释实验测量的同位素效应的方式。他们还指出了同位素效应在催化中的新应用,例如用于量化吸附物的覆盖率以及阐明催化剂表面的吸附和活性位点。
更新日期:2020-02-13
中文翻译:

覆盖率如何影响过渡金属上H2 / D2的解离吸附的热力学和动力学同位素效应
同位素效应极大地增进了我们对非均相催化中化学反应的理解。尽管已广泛使用它们,但对于现实的反应条件(例如表面被吸附物的覆盖范围)如何影响它们的理解仍然有限。在这里,我们研究氢(H)的覆盖对H 2 / D 2的热力学和动力学同位素效应的影响在催化相关的温度范围内,在12种过渡金属:Ag,Au,Co,Cu,Fe,Ir,Ni,Re,Pd,Pt,Rh和Ru的密排,开放和阶梯状表面上发生解离吸附。通过第一原理密度泛函理论计算,我们发现增加覆盖率具有两个作用:i)可能会改变首选的吸附位点和过渡态的几何形状,ii)由于H原子之间的相互作用而增加了被吸附H的振动频率。根据经验,对于大多数我们研究的系统,同位素效应的绝对值随覆盖率的增加而减小,表明稳定性的相对变化有利于D取代的最小值和过渡态。这可能是由于后一因素的持续影响,后者影响了所有结构。较高的温度降低了这些降低的幅度。我们的发现为覆盖机制影响同位素效应的纳米尺度机理提供了见识,而纳米机制将影响我们解释实验测量的同位素效应的方式。他们还指出了同位素效应在催化中的新应用,例如用于量化吸附物的覆盖率以及阐明催化剂表面的吸附和活性位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号