当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding 125Te NMR chemical shifts in disymmetric organo-telluride compounds from natural chemical shift analysis.
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9cp05934b Ewa Pietrasiak 1 , Christopher P Gordon 1 , Christophe Copéret 1 , Antonio Togni 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9cp05934b Ewa Pietrasiak 1 , Christopher P Gordon 1 , Christophe Copéret 1 , Antonio Togni 1
Affiliation
|
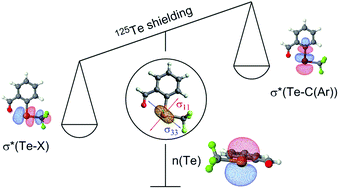
Organotellurium compounds of general formula X-Te-R display a broad range of chemical shifts that are very sensitive to the X and R substituents. In order to link the 125Te chemical shift of a series of perfluoroalkyl aryl tellurides to their electronic structure, the chemical shielding tensors of the 125Te nuclei were calculated by density functional theory (DFT) and further analyzed by a decomposition into contributions of natural localized molecular orbitals (NLMOs). The analysis indicated that the variation in 125Te chemical shifts in molecules 1-13 is mainly due to the magnetic coupling of the tellurium p-character lone pair with antibonding orbitals perpendicular to it {σ*(Te-X) and σ*(Te-C(Ar))} upon action of an external magnetic field. The strength of the coupling is affected by electronic properties of the X-substituents, polarization of the antibonding orbitals and presence of secondary interactions perturbing the energy of these orbitals. The lower in energy and the more polarized towards tellurium the antibonding orbitals are, the stronger is the coupling and the more deshielded the tellurium nucleus.
中文翻译:

通过自然化学位移分析了解双对称有机碲化合物中的125Te NMR化学位移。
通式X-Te-R的有机碲化合物显示出对X和R取代基非常敏感的广泛化学位移。为了将一系列全氟烷基芳基碲化物的125Te化学位移与其电子结构联系起来,通过密度泛函理论(DFT)计算了125Te核的化学屏蔽张量,并通过分解为自然局部分子轨道的贡献进行了进一步分析(NLMO)。分析表明,分子1-13中125Te化学位移的变化主要是由于碲p字符孤对与与其垂直的反键轨道{σ*(Te-X)和σ*(Te- C(Ar))}受外部磁场作用。耦合的强度受X取代基的电子特性影响,反键轨道的极化和次级相互作用的存在扰乱了这些轨道的能量。反键轨道的能量越低,并且对碲的极化程度越高,则耦合越强,碲原子核的屏蔽程度就越高。
更新日期:2020-01-13
中文翻译:

通过自然化学位移分析了解双对称有机碲化合物中的125Te NMR化学位移。
通式X-Te-R的有机碲化合物显示出对X和R取代基非常敏感的广泛化学位移。为了将一系列全氟烷基芳基碲化物的125Te化学位移与其电子结构联系起来,通过密度泛函理论(DFT)计算了125Te核的化学屏蔽张量,并通过分解为自然局部分子轨道的贡献进行了进一步分析(NLMO)。分析表明,分子1-13中125Te化学位移的变化主要是由于碲p字符孤对与与其垂直的反键轨道{σ*(Te-X)和σ*(Te- C(Ar))}受外部磁场作用。耦合的强度受X取代基的电子特性影响,反键轨道的极化和次级相互作用的存在扰乱了这些轨道的能量。反键轨道的能量越低,并且对碲的极化程度越高,则耦合越强,碲原子核的屏蔽程度就越高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号