当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissecting intermolecular interactions in the condensed phase of ibuprofen and related compounds: the specific role and quantification of hydrogen bonding and dispersion forces.
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-03-04 , DOI: 10.1039/c9cp06641a V N Emel'yanenko 1 , P Stange 1 , J Feder-Kubis 2 , S P Verevkin 3 , R Ludwig 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-03-04 , DOI: 10.1039/c9cp06641a V N Emel'yanenko 1 , P Stange 1 , J Feder-Kubis 2 , S P Verevkin 3 , R Ludwig 4
Affiliation
|
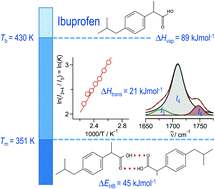
Ibuprofen is a well-established non-steroidal anti-inflammatory drug, inhibiting the prostaglandin-endoperoxide synthase. One of the key features defining the ibuprofen structure is the doubly intermolecular O-HO[double bond, length as m-dash]C hydrogen bond in cyclic dimers as know from carboxylic acids and confirmed by X-ray analysis. Until now, there was neither information about the vaporization enthalpy of ibuprofen nor about how this thermal property is determined by the subtle balance between different types of intermolecular interaction. In this study we derive the vaporization enthalpy of ibuprofen from thermochemical experiments to be . We dissected the hydrogen bond energy, EHB = 45.0 kJ mol-1, exclusively from measured vaporization enthalpies of related aliphatic carboxylic acids, their homomorph methyl esters and alkyl acetates, respectively. This contribution from hydrogen bonding could be confirmed almost quantitatively from quantum chemical calculations of ibuprofen clusters, which also suggest dispersion interaction of similar order (Edisp = 47 kJ mol-1). Following the full analysis of the gas-vapor transition enthalpy, we studied the changing structural components from the solid to the liquid phase of ibuprofen by means of Attenuated Total Reflection Infrared (ATR-IR) spectroscopy. The cyclic dimers as observed in the X-ray patterns are essentially preserved in the liquid state just above the melting point. However, with increasing temperature the doubly hydrogen-bonded cyclic dimers are replaced by singly hydrogen-bonded linear dimers in the liquid ibuprofen. The transfer enthalpy from the temperature-dependent equilibria of both dimers as obtained from the IR intensity ratios of the vibrational bands quantifies for the first time the energy of the released, single hydrogen bond to be EHB = 21.0 kJ mol-1. Overall, we show that a combination of thermodynamics, infrared spectroscopy and quantum chemistry provides quantification and detailed understanding of structure and molecular interaction in ibuprofen and related compounds.
中文翻译:

在布洛芬和相关化合物的缩合相中解析分子间的相互作用:氢键和分散力的特定作用和定量。
布洛芬是一种行之有效的非甾体类抗炎药,可抑制前列腺素-内过氧化物合酶。定义布洛芬结构的关键特征之一是环状二聚体中的双分子间O-HO [双键,长度为m-C]氢键,从羧酸中获知并通过X射线分析证实。到目前为止,还没有关于布洛芬的汽化焓的信息,也没有关于如何通过不同类型的分子间相互作用之间的微妙平衡确定该热性质的信息。在这项研究中,我们从热化学实验推导布洛芬的汽化焓为。我们仅从相关脂肪族羧酸,它们的同型甲酯和乙酸烷基酯的汽化焓测量值中解剖了氢键能EHB = 45.0 kJ mol-1。分别。氢键的这种贡献几乎可以从布洛芬簇的量子化学计算中定量地证实,这也表明相似数量级的分散相互作用(Edisp = 47 kJ mol-1)。在对气体-蒸气跃迁焓进行全面分析之后,我们通过衰减全反射红外(ATR-IR)光谱研究了布洛芬从固相到液相的变化结构组成。在X射线图上观察到的环状二聚体基本上保持在刚好高于熔点的液态。然而,随着温度的升高,液态布洛芬中的氢键合的环状二聚体被氢键合的线性二聚体所取代。从振动带的红外强度比获得的两个二聚体的温度依赖性平衡所产生的转移焓首次将释放的单个氢键的能量定量为EHB = 21.0 kJ mol-1。总的来说,我们表明热力学,红外光谱和量子化学的结合提供了布洛芬和相关化合物中结构和分子相互作用的定量和详细理解。
更新日期:2020-03-05
中文翻译:

在布洛芬和相关化合物的缩合相中解析分子间的相互作用:氢键和分散力的特定作用和定量。
布洛芬是一种行之有效的非甾体类抗炎药,可抑制前列腺素-内过氧化物合酶。定义布洛芬结构的关键特征之一是环状二聚体中的双分子间O-HO [双键,长度为m-C]氢键,从羧酸中获知并通过X射线分析证实。到目前为止,还没有关于布洛芬的汽化焓的信息,也没有关于如何通过不同类型的分子间相互作用之间的微妙平衡确定该热性质的信息。在这项研究中,我们从热化学实验推导布洛芬的汽化焓为。我们仅从相关脂肪族羧酸,它们的同型甲酯和乙酸烷基酯的汽化焓测量值中解剖了氢键能EHB = 45.0 kJ mol-1。分别。氢键的这种贡献几乎可以从布洛芬簇的量子化学计算中定量地证实,这也表明相似数量级的分散相互作用(Edisp = 47 kJ mol-1)。在对气体-蒸气跃迁焓进行全面分析之后,我们通过衰减全反射红外(ATR-IR)光谱研究了布洛芬从固相到液相的变化结构组成。在X射线图上观察到的环状二聚体基本上保持在刚好高于熔点的液态。然而,随着温度的升高,液态布洛芬中的氢键合的环状二聚体被氢键合的线性二聚体所取代。从振动带的红外强度比获得的两个二聚体的温度依赖性平衡所产生的转移焓首次将释放的单个氢键的能量定量为EHB = 21.0 kJ mol-1。总的来说,我们表明热力学,红外光谱和量子化学的结合提供了布洛芬和相关化合物中结构和分子相互作用的定量和详细理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号