当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Protecting group tuning of the enantioselectivity in Strecker reactions of trifluoromethyl ketimines to synthesize quaternary α-trifluoromethyl amino nitriles by ion pair catalysis.
Chemical Communications ( IF 4.3 ) Pub Date : 2020-01-14 , DOI: 10.1039/c9cc09151c Mengyuan Du 1 , Longhui Yu 2 , Ting Du 2 , Zhaokun Li 2 , Yueyang Luo 2 , Xiangyu Meng 2 , Zhengtao Tian 2 , Changwu Zheng 3 , Weiguo Cao 1 , Gang Zhao 2
Chemical Communications ( IF 4.3 ) Pub Date : 2020-01-14 , DOI: 10.1039/c9cc09151c Mengyuan Du 1 , Longhui Yu 2 , Ting Du 2 , Zhaokun Li 2 , Yueyang Luo 2 , Xiangyu Meng 2 , Zhengtao Tian 2 , Changwu Zheng 3 , Weiguo Cao 1 , Gang Zhao 2
Affiliation
|
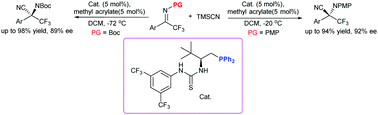
An enantioselective Strecker reaction to construct trifluoromethylated quaternary stereocenters with N-PMP and unexplored N-Boc trifluoromethyl ketimines catalyzed using an organophosphine dual-reagent catalyst has been developed. The enantioselectivities of the corresponding products with the same catalyst could be switched by using different N-protecting groups (N-PMP or N-Boc). The trifluoromethyl amino nitriles were obtained in high yield and high enantioselectivity in a short time and could be easily converted to a variety of useful trifluoromethyl-containing compounds.
中文翻译:

N保护基对三氟甲基酮亚胺在Strecker反应中通过离子对催化合成α-三氟甲基氨基腈的对映选择性的调节。
已开发出一种对映选择性斯特雷克反应,该反应可利用有机膦双试剂催化剂与N-PMP和未探索的N-Boc三氟甲基酮亚胺构建三氟甲基化的季立体中心。可以通过使用不同的N保护基(N-PMP或N-Boc)来切换相应产物与相同催化剂的对映选择性。在短时间内以高收率和高对映选择性获得了三氟甲基氨基腈,并且可以容易地将其转化为多种有用的含三氟甲基的化合物。
更新日期:2020-01-14
中文翻译:

N保护基对三氟甲基酮亚胺在Strecker反应中通过离子对催化合成α-三氟甲基氨基腈的对映选择性的调节。
已开发出一种对映选择性斯特雷克反应,该反应可利用有机膦双试剂催化剂与N-PMP和未探索的N-Boc三氟甲基酮亚胺构建三氟甲基化的季立体中心。可以通过使用不同的N保护基(N-PMP或N-Boc)来切换相应产物与相同催化剂的对映选择性。在短时间内以高收率和高对映选择性获得了三氟甲基氨基腈,并且可以容易地将其转化为多种有用的含三氟甲基的化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号