The Lancet HIV ( IF 12.8 ) Pub Date : 2020-01-03 , DOI: 10.1016/s2352-3018(19)30372-8 Dirk Schürmann 1 , Deanne Jackson Rudd 2 , Saijuan Zhang 2 , Inge De Lepeleire 3 , Martine Robberechts 3 , Evan Friedman 2 , Christian Keicher 4 , Andreas Hüser 4 , Jörg Hofmann 5 , Jay A Grobler 2 , S Aubrey Stoch 2 , Marian Iwamoto 2 , Randolph P Matthews 2
|
Background
Islatravir (also known as ISL and MK-8591) is a unique nucleoside reverse transcriptase translocation inhibitor in clinical development for treatment of people with HIV-1 infection. In preclinical studies, intracellular islatravir-triphosphate exhibits a long half-life and prolonged virological effects. In this study, we aimed to assess islatravir safety, pharmacokinetics, and antiretroviral activity in treatment-naive adults with HIV-1 infection.
Methods
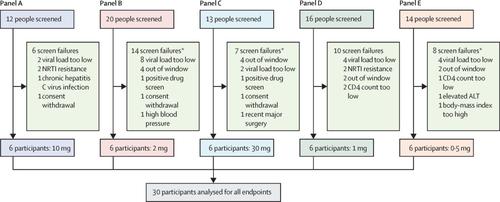
This open-label, consecutive-panel, phase 1b trial was done at Charité Research Organisation (Berlin, Germany) and included men and women (aged 18–60 years, inclusive) with HIV-1 infection who were ART naive. Participants were required to have plasma HIV-1 RNA counts of at least 10 000 copies per mL within 30 days before the trial treatment phase, without evidence of resistance to nucleoside reverse transcriptase inhibitors. Participants were enrolled in one of five consecutive dosing panels, receiving a single oral dose of islatravir (0·5–30 mg). The primary outcomes were safety and tolerability of islatravir and change from baseline in HIV-1 plasma RNA; secondary outcomes were islatravir plasma and islatravir-triphosphate intracellular pharmacokinetics. We obtained descriptive safety and pharmacokinetics statistics, and estimated efficacy results from a longitudinal data analysis model. This study is registered with ClinicalTrials.gov, NCT02217904, and EudraCT, 2014-002192-28.
Findings
Between Sept 17, 2015, and May 11, 2017, we enrolled 30 participants (six per panel). Islatravir was generally well tolerated. 27 (90%) participants had 60 adverse events after receipt of drug, of which 21 (35%) were deemed to be drug related. The most common (n>1) drug-related adverse events were headache (in nine [30%] participants) and diarrhoea (in two [7%]). No serious adverse events were reported, and no participants discontinued due to an adverse event. Plasma islatravir pharmacokinetics and intracellular islatravir-triphosphate pharmacokinetics were approximately dose proportional. The islatravir-triphosphate intracellular half-life was 78·5–128·0 h. Least-squares mean HIV-1 RNA at 7 days after dose decreased from 1·67 log10 copies per mL (95% CI 1·42–1·92) at 10 mg dose to 1·20 log10 copies per mL (0·95–1·46) at 0·5 mg dose. No genetic changes consistent with development of viral resistance were detected.
Interpretation
Single doses of islatravir as low as 0·5 mg significantly suppressed HIV-1 RNA by more than 1·0 log at day 7 in treatment-naive adults with HIV-1 infection and were generally well tolerated, supporting the further development of islatravir as a flexible-dose treatment for individuals with HIV-1 infection.
Funding
Merck Sharp & Dohme Corp, a subsidiary of Merck & Co Inc, Kenilworth, NJ, USA.
中文翻译:

新型单核苷酸逆转录酶易位抑制剂伊曲拉韦(ISL,MK-8591)的安全性,药代动力学和抗逆转录病毒活性-panel试用版。
背景
Islatravir(也称为ISL和MK-8591)是在临床开发中用于治疗HIV-1感染者的独特核苷逆转录酶易位抑制剂。在临床前研究中,细胞内三磷酸依斯曲韦具有长的半衰期和延长的病毒学作用。在这项研究中,我们旨在评估在未接受过HIV-1感染的未接受治疗的成年人中依曲拉韦的安全性,药代动力学和抗逆转录病毒活性。
方法
这项公开标签,连续面板的1b期试验是在Charité研究组织(德国柏林)进行的,研究对象包括天真的抗HIV-1感染的男女(年龄在18至60岁之间,包括18至60岁)。在试验治疗阶段之前的30天内,要求参与者的血浆HIV-1 RNA计数至少为每毫升10000拷贝,而没有证据表明对核苷逆转录酶抑制剂有抗性。参加者参加了五个连续的剂量评估小组之一,接受单次口服剂量的依曲他韦(0·5–30 mg)。主要结果是伊曲拉韦的安全性和耐受性以及HIV-1血浆RNA相对于基线的变化。次要结果是血浆伊曲特拉韦和三磷酸依曲韦胞内药代动力学。我们获得了描述性的安全性和药代动力学统计数据,并根据纵向数据分析模型估算出疗效。该研究已在ClinicalTrials.gov,NCT02217904和EudraCT进行了注册,2014-002192-28。
发现
在2015年9月17日至2017年5月11日之间,我们招募了30名参与者(每个小组六名)。Islatravir一般耐受性良好。27(90%)名参与者在服药后发生了60次不良事件,其中21名(35%)被认为与毒品有关。最常见的(n> 1)与药物相关的不良事件为头痛(9名[30%]参与者)和腹泻(2名[7%])。没有报告严重的不良事件,也没有参与者因不良事件而中断。血浆依斯拉韦的药代动力学和细胞内依斯拉韦三磷酸的药代动力学是近似剂量比例的。依特拉韦三磷酸的细胞内半衰期为78·5-128·0 h。最小二乘剂量后7天的平均HIV-1 RNA从10 mg剂量下的1· 67 log 10拷贝/ mL(95%CI 1·42-1·92)降低至1·20 log 10每毫升(0·95-1·46)的拷贝数为0·5 mg。未检测到与病毒抗性发展一致的遗传变化。
解释
在未接受治疗的初次感染HIV-1的成年人中,低剂量的Islatravir单次剂量低至0·5 mg即可在第7天显着抑制HIV-1 RNA超过1·0 log,并且总体耐受性良好,支持islatravir的进一步发展针对HIV-1感染者的灵活剂量治疗。
资金
默克夏普公司(Merck Sharp&Dohme Corp),默克公司(Merck&Co Inc),美国新泽西州Kenilworth的子公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号