The Lancet HIV ( IF 16.1 ) Pub Date : 2020-01-03 , DOI: 10.1016/s2352-3018(19)30322-4 Marta C Nunes 1 , Clare L Cutland 1 , Andrew Moultrie 1 , Stephanie Jones 1 , Justin R Ortiz 2 , Kathleen M Neuzil 2 , Keith P Klugman 3 , Eric A F Simões 4 , Adriana Weinberg 5 , Shabir A Madhi 1 ,
|
Background
Standard-dose, seasonal, trivalent, inactivated influenza vaccine induces moderate-to-low haemagglutination-inhibition antibody responses in people living with HIV. This study assessed the immunogenicity and safety of different dosing schedules of inactivated influenza vaccine in pregnant women living with HIV in South Africa.
Methods
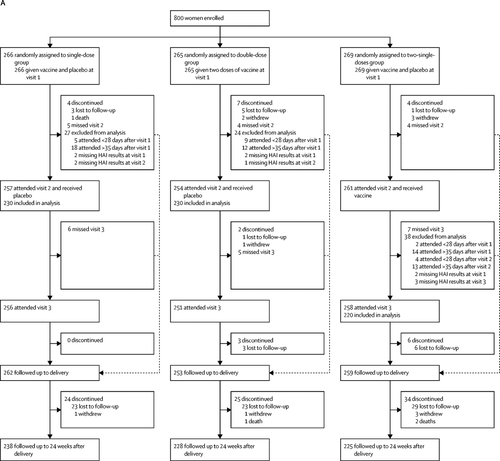
In this double-blind, randomised, controlled trial, we recruited pregnant women with HIV from seven antenatal clinics in Soweto, South Africa. Pregnant women were eligible if they were aged 18–38 years, infected with HIV, and had an estimated gestational age of 12–36 weeks. Women were randomly assigned (1:1:1), using a computer-generated randomisation list, to receive inactivated influenza vaccine containing 15 μg of each of the three seasonal influenza strains for that year, as a single dose, a double dose, or two single doses 1 month apart. Participants and study personnel were masked to group allocation. Haemagglutination-inhibition antibody responses were measured for all groups in the mothers at enrolment and at 1 month after each vaccine dose, and in the single-dose and double-dose groups within 7 days of birth in the neonates. Immunogenicity analyses only included women with visits 28–35 days apart and infants who were born at least 28 days after maternal immunisation. The primary was seroconversion rate to each of the vaccine strains in the mothers 1 month after completion of the dosing schedule, and the primary safety outcomes were frequency of local and systemic reactions. Safety was assessed in mothers and infants until 24 weeks post partum and analysed in all participants who received at least one dose of vaccine. This study is registered with ClinicalTrials.gov, NCT01527825, and is closed to accrual.
Findings
Between Feb 11, and June 6, 2013, 800 pregnant women living with HIV were enrolled and randomly assigned to the single-dose (n=266), double-dose (n=265), or two-single-doses (n=269) group. In the analysable population, seroconversion rates in mothers 1 month after the final vaccine dose were significantly higher in the double-dose group (n=230; ranging from 29% to 65% for the three vaccine strains) than in the single-dose group (n=230; ranging from 18% to 49%; p≤0·019 for the three vaccine strains), but were similar between the two-single-doses group (n=220; ranging from 23% to 52%) and the single-dose group (p≥0·20 for the three vaccine strains). Safety outcomes were similar in the three groups, except for more injection-site reactions in recipients in the double-dose group.
Interpretation
A regimen of double-dose inactivated influenza vaccine gave slightly greater immunogenicity than did a single-dose regimen in pregnant women living with HIV. However, immunogenicity in the double-dose group was still lower than historical data from the same setting in pregnant women without HIV. More immunogenic vaccines are needed for pregnant women living with HIV to enhance transplacental transfer of vaccine-induced protective antibodies to their newborn infants.
Funding
Bill & Melinda Gates Foundation.
中文翻译:

艾滋病毒孕妇三剂量灭活流感疫苗不同给药方案的免疫原性和安全性:一项随机对照试验。
背景
标准剂量的季节性三价灭活流感疫苗会在HIV感染者中引起中度至低度的血凝抑制抗体反应。这项研究评估了南非灭活艾滋病毒孕妇中不同剂量灭活流感疫苗的免疫原性和安全性。
方法
在这项双盲,随机,对照试验中,我们从南非索韦托的七个产前诊所招募了艾滋病毒孕妇。如果孕妇年龄在18-38岁之间,感染了HIV,且估计的孕龄为12-36周,则符合资格。使用计算机生成的随机列表将妇女随机分配(1:1:1:1),以当年三种剂量的季节性流感毒株中的每种15μg接种灭活的流感疫苗,以单剂量,双剂量或两次单剂,间隔1个月。参与者和研究人员被掩盖到小组分配中。在入组时和每次疫苗接种后1个月,对母亲的所有组以及新生儿出生后7天内的单剂量和双剂量组均测量了血凝抑制抗体反应。免疫原性分析仅包括相隔28-35天就诊的妇女以及在母体免疫后至少28天出生的婴儿。在给药方案完成后的1个月中,母亲的每种疫苗株的血清学转化率是主要因素,主要的安全性结果是局部和全身反应的频率。在产后24周之前对母亲和婴儿的安全性进行了评估,并对接受至少一剂疫苗的所有参与者进行了安全性分析。该研究已在ClinicalTrials.gov(NCT01527825)上进行了注册,目前尚未公开。主要的安全性结果是局部和全身反应的频率。在产后24周之前对母亲和婴儿的安全性进行了评估,并对接受至少一剂疫苗的所有参与者进行了安全性分析。该研究已在ClinicalTrials.gov(NCT01527825)上进行了注册,目前尚未公开。主要的安全性结果是局部和全身反应的频率。在产后24周之前对母亲和婴儿的安全性进行了评估,并对接受至少一剂疫苗的所有参与者进行了安全性分析。该研究已在ClinicalTrials.gov(NCT01527825)上进行了注册,目前尚未公开。
发现
在2013年2月11日至6月6日之间,我们招募了800名HIV孕妇,并随机分配给单剂量(n = 266),双剂量(n = 265)或两剂(n = 269)组。在可分析人群中,双剂量组的母亲在最终疫苗接种后1个月的血清转化率显着高于单剂量组(n = 230;三种疫苗株的范围从29%至65%)。 (n = 230;三种疫苗株的范围为18%至49%;p≤0·019),但在两个单剂量组之间相似(n = 220;范围为23%至52%)和单剂量组(三种疫苗株p≥0·20)。三组的安全性结果相似,除了双剂量组中更多的注射部位反应。
解释
在感染艾滋病毒的孕妇中,双剂量灭活流感疫苗的方案具有比单剂量方案稍大的免疫原性。但是,双剂量组的免疫原性仍低于没有HIV的孕妇在相同环境下的历史数据。感染艾滋病毒的孕妇需要更多的免疫原性疫苗,以增强疫苗诱导的保护性抗体经胎盘向新生婴儿的转移。
资金
比尔和梅琳达·盖茨基金会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号