当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Equilibrium thickness of foam films and adsorption of ions at surfaces: Water and aqueous solutions of sodium chloride, hydrochloric acid, and sodium hydroxide.
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.jcis.2019.12.042 Hidemi Iyota 1 , Rumen Krastev 2
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.jcis.2019.12.042 Hidemi Iyota 1 , Rumen Krastev 2
Affiliation
|
HYPOTHESIS
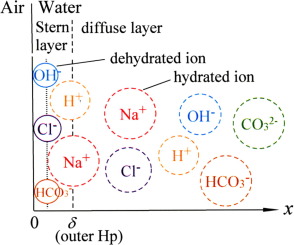
The origin of negative surface charge at water/air interface is still not clear. The most probable origin is specific adsorption of OH- ions. From diffuse layer potential, we can evaluate the surface density of ions in the Stern layer which can be a measure for the specific adsorption of ions and determines whether the surface charge is solely due to the specific adsorption of OH- ions.
EXPERIMENTS
Equilibrium thickness of foam films of pure water and aqueous solutions of NaCl, HCl, and NaOH was measured as a function of disjoining pressure for water and as a function of concentration for the aqueous solutions at 298.15 K. Quartz-glass cells thoroughly cleaned and immersed in pure water before use were used for the measurement.
FINDINGS
Application of a modified Poisson-Boltzmann equation to the equilibrium film thickness gave the diffuse layer potential and the surface density of ions in the Stern layer. From the concentration dependence of the surface density, it was concluded that not only OH- ions but also Cl- ions and HCO3- and/or CO32- ions adsorb specifically at the water/air interface.
中文翻译:

泡沫膜的平衡厚度和表面离子的吸附:水和氯化钠,盐酸和氢氧化钠的水溶液。
假设水/空气界面的负表面电荷的起因仍然不清楚。最可能的原因是特定的OH离子吸附。从扩散层的电势,我们可以评估斯特恩层中离子的表面密度,这可以衡量离子的特定吸附,并确定表面电荷是否仅是由于OH-离子的特定吸附。实验测量纯水和NaCl,HCl和NaOH水溶液的泡沫膜的平衡厚度,该值取决于水的分离压力和298.15 K下水溶液的浓度的函数。彻底清洁并清洗了石英玻璃电池使用前将其浸入纯净水中进行测量。发现将修正的Poisson-Boltzmann方程应用于平衡膜厚度,可以得出扩散层电势和斯特恩层中离子的表面密度。从表面密度的浓度依赖性可以得出结论,不仅OH-离子而且Cl-离子和HCO3-和/或CO32-离子都特别吸附在水/空气界面。
更新日期:2020-01-04
中文翻译:

泡沫膜的平衡厚度和表面离子的吸附:水和氯化钠,盐酸和氢氧化钠的水溶液。
假设水/空气界面的负表面电荷的起因仍然不清楚。最可能的原因是特定的OH离子吸附。从扩散层的电势,我们可以评估斯特恩层中离子的表面密度,这可以衡量离子的特定吸附,并确定表面电荷是否仅是由于OH-离子的特定吸附。实验测量纯水和NaCl,HCl和NaOH水溶液的泡沫膜的平衡厚度,该值取决于水的分离压力和298.15 K下水溶液的浓度的函数。彻底清洁并清洗了石英玻璃电池使用前将其浸入纯净水中进行测量。发现将修正的Poisson-Boltzmann方程应用于平衡膜厚度,可以得出扩散层电势和斯特恩层中离子的表面密度。从表面密度的浓度依赖性可以得出结论,不仅OH-离子而且Cl-离子和HCO3-和/或CO32-离子都特别吸附在水/空气界面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号