当前位置:
X-MOL 学术
›
Mater. Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controllable and stable organometallic redox mediators for lithium oxygen batteries
Materials Horizons ( IF 12.2 ) Pub Date : 2019-08-29 , DOI: 10.1039/c9mh01043b Won-Jin Kwak 1, 2, 3, 4 , Atif Mahammed 5, 6, 7, 8 , Hun Kim 1, 2, 3, 4 , Trung Thien Nguyen 1, 2, 3, 4 , Zeev Gross 5, 6, 7, 8 , Doron Aurbach 8, 9, 10, 11 , Yang-Kook Sun 1, 2, 3, 4, 12
Materials Horizons ( IF 12.2 ) Pub Date : 2019-08-29 , DOI: 10.1039/c9mh01043b Won-Jin Kwak 1, 2, 3, 4 , Atif Mahammed 5, 6, 7, 8 , Hun Kim 1, 2, 3, 4 , Trung Thien Nguyen 1, 2, 3, 4 , Zeev Gross 5, 6, 7, 8 , Doron Aurbach 8, 9, 10, 11 , Yang-Kook Sun 1, 2, 3, 4, 12
Affiliation
|
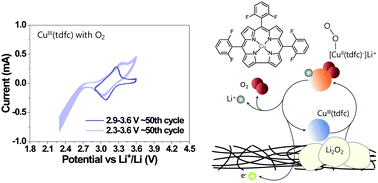
The use of electrocatalysis in lithium–oxygen batteries is mandatory for reducing the over-potentials of the oxygen evolution reaction (OER), below the levels that endanger the anodic stability of the electrolyte solutions and the carbon electrodes. The most effective catalysts for the OER are solubilized redox mediators that may be oxidized at relatively low potentials, but still capable of oxidizing Li2O2 back to molecular oxygen. Since for the effective and long-term utilization of redox mediators in lithium–oxygen cells a clear evaluation of their stability is essential, we have developed a useful methodology for that purpose. This revealed, quite surprisingly, that most commonly used redox mediators are unstable in lithium–oxygen cells, even under argon atmosphere and without being in contact with Li anodes. Using the abovementioned methodology for evaluating efficiency, we now introduce corrole-chelated metal complexes as stable redox mediators in lithium oxygen batteries. This was achieved by taking advantage of the facile methods for introducing changes in the corrole ligands and by choosing properly the central transition metal cation, two aspects that allow for adjusting the redox properties of the metal complexes for the operative voltage window. We outline further directions and believe that this work will promote optimized selection of redox mediators for lithium–oxygen batteries.
中文翻译:

用于锂氧电池的可控且稳定的有机金属氧化还原介体
在锂氧电池中必须使用电催化作用,以将氧释放反应(OER)的过电势降低到危害电解质溶液和碳电极的阳极稳定性的水平以下。OER最有效的催化剂是可溶解的氧化还原介体,可以在相对低的电位下氧化,但仍然能够氧化Li 2 O 2。回到分子氧。为了有效地长期使用氧化还原介体在锂氧电池中,对它们的稳定性进行清晰的评估至关重要,因此我们为此目的开发了一种有用的方法。出乎意料的是,这表明,即使在氩气气氛下且未与锂阳极接触,最常用的氧化还原介体在锂氧电池中也是不稳定的。使用上述方法评估效率,我们现在将螯合螯合的金属络合物作为稳定的氧化还原介体引入锂氧电池中。这是通过利用用于引入在corrole配体的变化容易的方法的优点,并通过选择适当的中心过渡金属阳离子来实现,有两个方面可以针对工作电压窗口调整金属配合物的氧化还原特性。我们概述了进一步的方向,并相信这项工作将促进锂氧电池氧化还原介体的优化选择。
更新日期:2020-01-04
中文翻译:

用于锂氧电池的可控且稳定的有机金属氧化还原介体
在锂氧电池中必须使用电催化作用,以将氧释放反应(OER)的过电势降低到危害电解质溶液和碳电极的阳极稳定性的水平以下。OER最有效的催化剂是可溶解的氧化还原介体,可以在相对低的电位下氧化,但仍然能够氧化Li 2 O 2。回到分子氧。为了有效地长期使用氧化还原介体在锂氧电池中,对它们的稳定性进行清晰的评估至关重要,因此我们为此目的开发了一种有用的方法。出乎意料的是,这表明,即使在氩气气氛下且未与锂阳极接触,最常用的氧化还原介体在锂氧电池中也是不稳定的。使用上述方法评估效率,我们现在将螯合螯合的金属络合物作为稳定的氧化还原介体引入锂氧电池中。这是通过利用用于引入在corrole配体的变化容易的方法的优点,并通过选择适当的中心过渡金属阳离子来实现,有两个方面可以针对工作电压窗口调整金属配合物的氧化还原特性。我们概述了进一步的方向,并相信这项工作将促进锂氧电池氧化还原介体的优化选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号