Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.micromeso.2020.109999 Hao Wu , Philippe Trens , Bernard Fraisse , Fabrice Salles , Jerzy Zajac
|
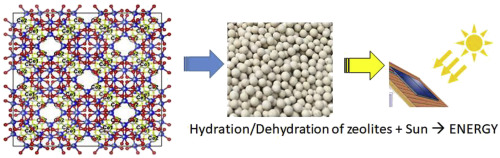
The potential use of commercially available 13X zeolite, modified by ion-exchange with cerium compensating cations possessing high charges and high hydration energies, has been tested in view of low-temperature storage of solar energy performed under mild operating conditions of low regeneration temperatures and low pressures of water vapour during the adsorption step. Structural and textural properties, sorption behaviour towards water vapour of three selected samples containing various proportions of Ce3+ and Ce4+ compensating cations and the pristine Na+-13X zeolite were studied by a variety of experimental techniques including Wavelength Dispersive X-Ray Fluorescence, Energy Dispersive X-ray Spectroscopy, X-ray diffraction, Thermogravimetric analysis, as well as measurements of the adsorption of gaseous nitrogen at 77 K and water vapour at 313 K. Based on the structure refinement procedure applied to the experimental XRD patterns, it was demonstrated that extra-framework cerium cations were preferentially located on sites I’ and II in dry and hydrated zeolites, showing relatively little dependence on the hydration level. Monte Carlo simulations were used to determine the limit values of the amount adsorbed and differential heat of adsorption, which could be obtained experimentally if the zeolite samples were completely dried. The potential of Ce-containing zeolites as adsorbents for the thermochemical energy storage was finally determined under flow conditions by firstly dehydrating samples at 353 K or 423 K and then saturating them at 296 K with water vapour at a mole fraction of 0.03. The choice of the operating conditions was decided so as to maintain the stability of the zeolite structure while taking the risk of reduced thermal performance of zeolite adsorbents undergoing incomplete regeneration-dehydration. Under such mild conditions, the modified 13X zeolites exhibited enhanced thermal performance in comparison with that of the pristine 13X, by releasing between 700 and 1100 kJ per kg of the adsorbent during a period of 6–8 h. Through a complementary study based on calorimetry measurements and molecular simulations, the understanding of the hydration-dehydration steps in Ce-exchanged zeolites and cation displacement upon hydration has allowed to establish the best compromise for the conditions of zeolite regeneration and saturation in the case of heat long-term storage applications.
中文翻译:

铈交换沸石的水化机理和吸附水蒸气时的放热性能,以支持其在温和的吸附剂再生和饱和条件下热化学存储能量中的潜在用途
考虑到在低再生温度和低温和运行条件下进行的低温太阳能存储,已经测试了通过离子交换与具有高电荷和高水化能的铈补偿阳离子进行改性的市售13X沸石的潜在用途。吸附步骤中水蒸气的压力。三种含有不同比例的Ce 3+和Ce 4+补偿阳离子以及原始Na +的样品的结构和织构特性,对水蒸气的吸附行为-13X沸石通过多种实验技术进行了研究,包括波长色散X射线荧光,能量色散X射线光谱法,X射线衍射,热重分析,以及在77 K和水蒸气下气态氮的吸附测量在313 K时。基于应用于实验XRD图案的结构优化程序,证明了骨架外的铈阳离子优先位于干燥和水合沸石中的I'和II位,对水合度的依赖性相对较小。使用蒙特卡洛模拟确定吸附量的极限值和吸附的差热,如果将沸石样品完全干燥,则可以通过实验获得极限值。最终,在流动条件下,首先通过在353 K或423 K下使样品脱水,然后在296 K下用摩尔分数为0.03的水蒸气使样品饱和,来确定含Ce沸石作为热化学能量存储吸附剂的潜力。确定操作条件的选择是为了维持沸石结构的稳定性,同时冒降低经历不完全再生脱水的沸石吸附剂热性能的风险。在这种温和的条件下,改性的13X沸石与原始13X相比,在6-8小时内释放出700至1100 kJ / kg的吸附剂,从而具有更高的热性能。通过基于量热法测量和分子模拟的补充研究,











































 京公网安备 11010802027423号
京公网安备 11010802027423号