当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Glycosylation and Gaucher Disease Mutation Allosterically Alter Active-Site Dynamics of Acid-β-Glucosidase
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-14 , DOI: 10.1021/acscatal.9b04404 Michael Gregory Souffrant , Xin-Qiu Yao , Mohamed Momin , Donald Hamelberg
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-14 , DOI: 10.1021/acscatal.9b04404 Michael Gregory Souffrant , Xin-Qiu Yao , Mohamed Momin , Donald Hamelberg
|
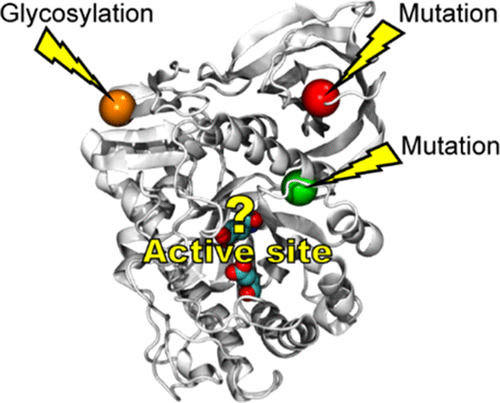
Allosteric regulations of the catalytic activity of enzymes in cellular processes remain poorly understood. Here, we advance the understanding of these critical processes by providing insights into the allosteric mechanism of acid-β-glucosidase, an enzyme associated with Gaucher disease. Glycosylation, a post-translational modification of proteins, is essential for in vivo catalytic activity and stability of acid-β-glucosidase. Asparagine 19, a glycosylation site that is located ∼30 Å from the active site, was observed to be glycosylated with either one or two N-acetyl-glucosamines by X-ray crystallography. In addition, N370S and L444P mutations, located ∼18 and 30 Å, respectively, away from the active site, cause type I Gaucher disease that is common among certain demographic groups. Using multiple microsecond-long molecular dynamics simulations, we evaluated the dynamics of the wild-type, glycosylated with one and two N-acetyl-glucosamines at N19 and the N370S and L444P variants of acid-β-glucosidase. Our results suggest that access to the substrate-binding pocket is controlled by the dynamics of loops surrounding the active site and allosterically regulated. Catalytic residues, E235 and E340, are less localized and sample more conformational states upon glycosylation of acid-β-glucosidase when compared to the wild-type and N370S variant. Our findings could help explain the allosteric effects of glycosylation on enzyme mechanisms, other disease-causing mutations in acid-β-glucosidase, and facilitate the design of drugs for the treatment of Gaucher disease.
中文翻译:

N-糖基化和戈谢病突变变构改变酸性β-葡萄糖苷酶的活性部位动力学
关于酶在细胞过程中的催化活性的变构调控仍然知之甚少。在这里,我们通过提供对酸性-β-葡萄糖苷酶(一种与高雪氏病相关的酶)的变构机制的深入了解,来增进对这些关键过程的理解。糖基化是蛋白质的翻译后修饰,对于酸性β-葡萄糖苷酶的体内催化活性和稳定性至关重要。通过X射线晶体学观察到天冬酰胺19是位于距活性位点约30埃处的糖基化位点,被一种或两种N-乙酰基-葡糖胺糖基化。此外,分别距活动位点约18和30Å的N370S和L444P突变引起某些人群中常见的I型Gaucher疾病。使用多个微秒长的分子动力学模拟,我们评估了在N19处被一种和两种N-乙酰基葡萄糖胺糖基化的野生型的动力学,以及酸性β-葡萄糖苷酶的N370S和L444P变体。我们的研究结果表明,接近底物结合袋的过程是由围绕活性位点的环的动力学控制的,并且通过变构调节。与野生型和N370S变体相比,酸-β-葡萄糖苷酶糖基化后,催化残基E235和E340的定位较少,构象状态更丰富。我们的发现可以帮助解释糖基化对酶机制的变构作用,酸-β-葡萄糖苷酶的其他致病突变,并有助于设计用于治疗高雪氏病的药物。在N19处被一个和两个N-乙酰基葡萄糖胺糖基化,以及酸性β-葡萄糖苷酶的N370S和L444P变体。我们的研究结果表明,接近底物结合袋的过程是由围绕活性位点的环的动力学控制的,并且通过变构调节。与野生型和N370S变体相比,酸-β-葡萄糖苷酶糖基化后,催化残基E235和E340的定位较少,构象状态更丰富。我们的发现可以帮助解释糖基化对酶机制的变构作用,酸-β-葡萄糖苷酶的其他致病突变,并有助于设计用于治疗高雪氏病的药物。在N19处被一个和两个N-乙酰基葡萄糖胺糖基化,以及酸性β-葡萄糖苷酶的N370S和L444P变体。我们的研究结果表明,接近底物结合袋的过程是由围绕活性位点的环的动力学控制的,并且通过变构调节。与野生型和N370S变体相比,酸-β-葡萄糖苷酶糖基化后,催化残基E235和E340的定位较少,构象状态更丰富。我们的发现可以帮助解释糖基化对酶机制的变构作用,酸-β-葡萄糖苷酶的其他致病突变,并有助于设计用于治疗高雪氏病的药物。我们的结果表明,接近底物结合口袋的过程是由围绕活性位点的环的动力学控制的,并且通过变构调节。与野生型和N370S变体相比,酸-β-葡萄糖苷酶糖基化后,催化残基E235和E340的定位较少,构象状态更丰富。我们的发现可以帮助解释糖基化对酶机制的变构作用,酸-β-葡萄糖苷酶的其他致病突变,并有助于设计用于治疗高雪氏病的药物。我们的结果表明,接近底物结合口袋的过程是由围绕活性位点的环的动力学控制的,并且通过变构调节。与野生型和N370S变体相比,酸-β-葡萄糖苷酶糖基化后,催化残基E235和E340的定位较少,构象状态更丰富。我们的发现可以帮助解释糖基化对酶机制的变构作用,酸-β-葡萄糖苷酶的其他致病突变,并有助于设计用于治疗高雪氏病的药物。
更新日期:2020-01-14
中文翻译:

N-糖基化和戈谢病突变变构改变酸性β-葡萄糖苷酶的活性部位动力学
关于酶在细胞过程中的催化活性的变构调控仍然知之甚少。在这里,我们通过提供对酸性-β-葡萄糖苷酶(一种与高雪氏病相关的酶)的变构机制的深入了解,来增进对这些关键过程的理解。糖基化是蛋白质的翻译后修饰,对于酸性β-葡萄糖苷酶的体内催化活性和稳定性至关重要。通过X射线晶体学观察到天冬酰胺19是位于距活性位点约30埃处的糖基化位点,被一种或两种N-乙酰基-葡糖胺糖基化。此外,分别距活动位点约18和30Å的N370S和L444P突变引起某些人群中常见的I型Gaucher疾病。使用多个微秒长的分子动力学模拟,我们评估了在N19处被一种和两种N-乙酰基葡萄糖胺糖基化的野生型的动力学,以及酸性β-葡萄糖苷酶的N370S和L444P变体。我们的研究结果表明,接近底物结合袋的过程是由围绕活性位点的环的动力学控制的,并且通过变构调节。与野生型和N370S变体相比,酸-β-葡萄糖苷酶糖基化后,催化残基E235和E340的定位较少,构象状态更丰富。我们的发现可以帮助解释糖基化对酶机制的变构作用,酸-β-葡萄糖苷酶的其他致病突变,并有助于设计用于治疗高雪氏病的药物。在N19处被一个和两个N-乙酰基葡萄糖胺糖基化,以及酸性β-葡萄糖苷酶的N370S和L444P变体。我们的研究结果表明,接近底物结合袋的过程是由围绕活性位点的环的动力学控制的,并且通过变构调节。与野生型和N370S变体相比,酸-β-葡萄糖苷酶糖基化后,催化残基E235和E340的定位较少,构象状态更丰富。我们的发现可以帮助解释糖基化对酶机制的变构作用,酸-β-葡萄糖苷酶的其他致病突变,并有助于设计用于治疗高雪氏病的药物。在N19处被一个和两个N-乙酰基葡萄糖胺糖基化,以及酸性β-葡萄糖苷酶的N370S和L444P变体。我们的研究结果表明,接近底物结合袋的过程是由围绕活性位点的环的动力学控制的,并且通过变构调节。与野生型和N370S变体相比,酸-β-葡萄糖苷酶糖基化后,催化残基E235和E340的定位较少,构象状态更丰富。我们的发现可以帮助解释糖基化对酶机制的变构作用,酸-β-葡萄糖苷酶的其他致病突变,并有助于设计用于治疗高雪氏病的药物。我们的结果表明,接近底物结合口袋的过程是由围绕活性位点的环的动力学控制的,并且通过变构调节。与野生型和N370S变体相比,酸-β-葡萄糖苷酶糖基化后,催化残基E235和E340的定位较少,构象状态更丰富。我们的发现可以帮助解释糖基化对酶机制的变构作用,酸-β-葡萄糖苷酶的其他致病突变,并有助于设计用于治疗高雪氏病的药物。我们的结果表明,接近底物结合口袋的过程是由围绕活性位点的环的动力学控制的,并且通过变构调节。与野生型和N370S变体相比,酸-β-葡萄糖苷酶糖基化后,催化残基E235和E340的定位较少,构象状态更丰富。我们的发现可以帮助解释糖基化对酶机制的变构作用,酸-β-葡萄糖苷酶的其他致病突变,并有助于设计用于治疗高雪氏病的药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号