当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing in Vitro Release Kinetics of Long-Acting Injectable Nanosuspensions via Flow-NMR Spectroscopy.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.molpharmaceut.9b00958 Nathan D Rudd 1 , Roy Helmy 2 , Peter G Dormer 3 , R Thomas Williamson 3 , W Peter Wuelfing 1 , Paul L Walsh 1 , Mikhail Reibarkh 3 , William P Forrest 4
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.molpharmaceut.9b00958 Nathan D Rudd 1 , Roy Helmy 2 , Peter G Dormer 3 , R Thomas Williamson 3 , W Peter Wuelfing 1 , Paul L Walsh 1 , Mikhail Reibarkh 3 , William P Forrest 4
Affiliation
|
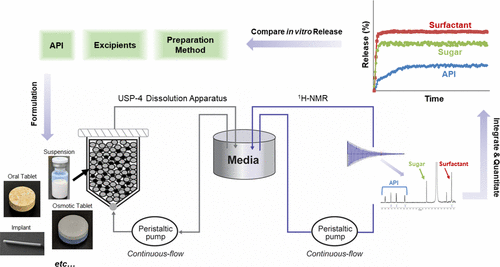
Novel treatment routes are emerging for an array of diseases and afflictions. Complex dosage forms, based on active pharmaceutical ingredients (APIs) with previously undesirable physicochemical characteristics, are becoming mainstream and actively pursued in various pipeline initiatives. To fundamentally understand how constituents in these dosage forms interact on a molecular level, analytical methods need to be developed that encompass selectivity and sensitivity requirements previously reserved for a myriad of in vitro techniques. The knowledge of precise chemical interactions between drugs and excipients in a dosage form can streamline formulation development and process screening capabilities through the identification of properties that influence rates and mechanisms of drug release in a cost-effective manner, relative to long-term in vivo studies. Through this work, a noncompendial in vitro release (IVR) method was developed that distinguished the presence of individual components in a complex crystalline nanosuspension environment. Doravirine was formulated as a series of long-acting injectable nanosuspensions with assorted excipients, using low- and high-energy wet media milling methods. IVR behavior of all formulation components were monitored using a robust continuous flow-through (CFT) dissolution setup (USP-4 apparatus) with on-line 1H NMR end-analysis (flow-NMR). Results from this investigation led to a better understanding of formulation parameter influences on nanosuspension stability, surface chemistry, and dissolution behavior. Flow-NMR can be applied to a broad range of dosage forms in which specific molecular interactions from the solution microenvironment require further insight to enhance product development capabilities.
中文翻译:

通过流式核磁共振波谱研究长效可注射纳米悬浮液的体外释放动力学。
针对一系列疾病和折磨的新型治疗途径正在兴起。基于具有以前不希望的理化特性的活性药物成分(API)的复杂剂型正成为主流,并在各种开发计划中得到了积极追求。为了从根本上理解这些剂型中的成分如何在分子水平上相互作用,需要开发一种分析方法,以涵盖以前为多种体外技术保留的选择性和敏感性要求。剂型中药物与赋形剂之间精确的化学相互作用的知识可通过识别以经济有效的方式影响药物释放速率和机制的特性,从而简化制剂开发和过程筛选的能力,相对于长期体内研究。通过这项工作,开发了一种非药物体外释放(IVR)方法,该方法可以区分复杂晶体纳米悬浮液环境中单个成分的存在。使用低能量和高能量湿介质研磨方法,将Doravirine配制成一系列长效可注射纳米悬浮液和各种赋形剂。使用具有在线1H NMR末端分析(flow-NMR)的强大的连续流通(CFT)溶出装置(USP-4装置),可以监控所有制剂组分的IVR行为。这项研究的结果使人们对配方参数对纳米悬浮液稳定性,表面化学和溶解行为的影响有了更好的了解。
更新日期:2020-01-15
中文翻译:

通过流式核磁共振波谱研究长效可注射纳米悬浮液的体外释放动力学。
针对一系列疾病和折磨的新型治疗途径正在兴起。基于具有以前不希望的理化特性的活性药物成分(API)的复杂剂型正成为主流,并在各种开发计划中得到了积极追求。为了从根本上理解这些剂型中的成分如何在分子水平上相互作用,需要开发一种分析方法,以涵盖以前为多种体外技术保留的选择性和敏感性要求。剂型中药物与赋形剂之间精确的化学相互作用的知识可通过识别以经济有效的方式影响药物释放速率和机制的特性,从而简化制剂开发和过程筛选的能力,相对于长期体内研究。通过这项工作,开发了一种非药物体外释放(IVR)方法,该方法可以区分复杂晶体纳米悬浮液环境中单个成分的存在。使用低能量和高能量湿介质研磨方法,将Doravirine配制成一系列长效可注射纳米悬浮液和各种赋形剂。使用具有在线1H NMR末端分析(flow-NMR)的强大的连续流通(CFT)溶出装置(USP-4装置),可以监控所有制剂组分的IVR行为。这项研究的结果使人们对配方参数对纳米悬浮液稳定性,表面化学和溶解行为的影响有了更好的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号