Journal of Chromatography B ( IF 2.8 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.jchromb.2019.121961 Muzaffar Iqbal , Essam Ezzeldin , Rashed Naji Herqash , Md. Khalid Anwer , Faizul Azam
|
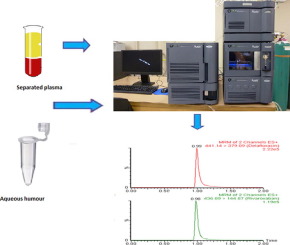
Acute bacterial skin and skin structure infections are one of the most frequent infectious disease requiring hospitalization for treatment. Delafloxacin is a clinically approved fluoroquinolone antibiotic for the treatment of ABSSSIs. In spite of being marketed since 2017, there is no published analytical method for quantification of delafloxacin in biological samples. Herein, a selective and sensitive UPLC-MS/MS method was developed and validated for quantitative analysis of delafloxacin in rat plasma and rabbit aqueous humour samples. The liquid liquid extraction (using ethyl acetate) was used for analyte extraction form rat plasma, whereas protein precipitation (acetonitrile) was used for aqueous humour samples preparations. An Acquity UPLC BEH C18 column was used for chromatographic separation of delafloxacin and internal standard (rivaroxaban). The mobile phase composition of acetonitrile (containing 0.1 % formic acid) and 10 mM ammonium acetate in ratio of 60:40 were used for sample elution at 300 µL/min flow rate. The electrospray ionization operated in positive modewas used for sample ionizationand detection of analyte and internal standard were performed by multiple reaction monitoring (MRM) mode. The MRM transitions were set to 441.14 > 379.09 and 436.89 > 144.87 for delafloxacin and internal standard, respectively. The method was validated as per USFDA guideline for bioanalytical method and all the evaluated parameters were within the acceptable ranges. The developed method in plasma was successfully used to analyze samples in pharmacokinetic study of newly developed stearic acid-chitosan solid lipid nanoparticles formulation of delafloxacin in rat.
中文翻译:

新型UPLC-MS / MS方法的开发和验证,用于定量测定血浆和房水中地拉氟沙星的药代动力学
急性细菌性皮肤和皮肤结构感染是需要住院治疗的最常见的传染病之一。德拉福沙星是一种临床认可的氟喹诺酮类抗生素,用于治疗ABSSSI。尽管自2017年以来开始销售,但尚未发布用于定量生物样品中地氟沙星的分析方法。在本文中,开发了一种选择性和灵敏的UPLC-MS / MS方法,并验证了该方法可用于定量分析大鼠血浆和兔房水样品中的去氟沙星。液液萃取(使用乙酸乙酯)用于从大鼠血浆中提取分析物,而蛋白质沉淀(乙腈)用于房水样品制备。Acquity UPLC BEH C 18色谱柱用于delafloxacin和内标(利伐沙班)的色谱分离。乙腈(含0.1%的甲酸)和10 mM乙酸铵(比例为60:40)的流动相组合物以300 µL / min的流速用于样品洗脱。以正模式操作的电喷雾电离用于样品电离,并通过多反应监测(MRM)模式进行分析物和内标的检测。地拉氟沙星和内标物的MRM过渡分别设置为441.14> 379.09和436.89> 144.87。该方法已按照美国食品药品管理局(USFDA)的生物分析方法指南进行了验证,所有评估参数均在可接受的范围内。











































 京公网安备 11010802027423号
京公网安备 11010802027423号