当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ferrous iron binding to epinephrine promotes the oxidation of iron and impedes activation of adrenergic receptors.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.freeradbiomed.2020.01.001 Jelena Korać Jačić 1 , Ljiljana Nikolić 2 , Dalibor M Stanković 3 , Miloš Opačić 1 , Milena Dimitrijević 1 , Danijela Savić 2 , Sanja Grgurić Šipka 4 , Ivan Spasojević 1 , Jelena Bogdanović Pristov 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.freeradbiomed.2020.01.001 Jelena Korać Jačić 1 , Ljiljana Nikolić 2 , Dalibor M Stanković 3 , Miloš Opačić 1 , Milena Dimitrijević 1 , Danijela Savić 2 , Sanja Grgurić Šipka 4 , Ivan Spasojević 1 , Jelena Bogdanović Pristov 1
Affiliation
|
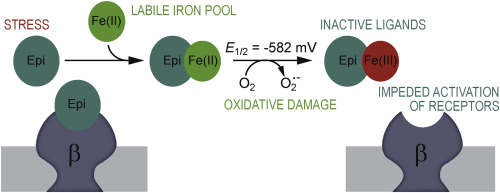
Upon release in response to stress, epinephrine (Epi) may interact with labile iron pool in human plasma with potentially important (patho)physiological consequences. We have shown that Epi and Fe3+ build stable 1:1 high-spin bidentate complex at physiological pH, and that Epi does not undergo degradation in the presence of iron. However, the interactions of Epi with the more soluble Fe2+, and the impact of iron on biological activity of Epi are still not known. Herein we showed that Epi and Fe2+ build colorless complex which is stable under anaerobic conditions. In the presence of O2, Epi promoted the oxidation of Fe2+ and the formation of Epi-Fe3+ complex. Cyclic voltammetry showed that mid-point potential of Epi-Fe2+ complex is very low (-582 mV vs. standard hydrogen electrode), which explains catalyzed oxidation of Fe2+. Next, we examined the impact of iron binding on biological performance of Epi using patch clamping in cell culture with constitutive expression of adrenergic receptors. Epi alone evoked an increase of outward currents, whereas Epi in the complex with Fe3+ did not. This implies that the binding of Epi to adrenergic receptors and their activation is prevented by the formation of complex with iron. Pro-oxidative activity of Epi-Fe2+ complex may represent a link between chronic stress and cardiovascular problems. On the other hand, labile iron could serve as a modulator of biological activity of ligands. Such interactions may be important in human pathologies that are related to iron overload or deficiency.
中文翻译:

铁与肾上腺素的结合促进铁的氧化并阻碍肾上腺素能受体的激活。
在响应压力而释放时,肾上腺素(Epi)可能与人体血浆中的不稳定铁池相互作用,从而可能产生重要的(病理)生理后果。我们已经显示Epi和Fe3 +在生理pH值下可建立稳定的1:1高纺丝双齿复合物,并且Epi在铁存在下不会发生降解。然而,Epi与更易溶的Fe2 +的相互作用以及铁对Epi的生物活性的影响仍然未知。在这里,我们表明Epi和Fe2 +生成无色络合物,在厌氧条件下稳定。在氧气存在下,Epi促进了Fe2 +的氧化和Epi-Fe3 +络合物的形成。循环伏安法显示Epi-Fe2 +络合物的中点电势非常低(相对于标准氢电极为-582 mV),这说明了Fe2 +的催化氧化。下一个,我们使用肾上腺素能受体的组成型表达在细胞培养中使用膜片钳研究了铁结合对Epi生物学性能的影响。单独的Epi引起外向电流的增加,而与Fe3 +形成的复合物中的Epi却没有。这暗示着通过与铁形成络合物阻止了Epi与肾上腺素能受体的结合及其活化。Epi-Fe2 +复合物的促氧化活性可能代表了慢性应激与心血管疾病之间的联系。另一方面,不稳定的铁可以充当配体生物活性的调节剂。这种相互作用在与铁超负荷或缺乏有关的人类病理中可能很重要。单独的Epi引起外向电流的增加,而与Fe3 +形成的复合物中的Epi却没有。这暗示着通过与铁形成络合物阻止了Epi与肾上腺素能受体的结合及其活化。Epi-Fe2 +复合物的促氧化活性可能代表了慢性应激与心血管疾病之间的联系。另一方面,不稳定的铁可以充当配体生物活性的调节剂。这种相互作用在与铁超负荷或缺乏有关的人类病理中可能很重要。单独的Epi引起外向电流的增加,而与Fe3 +形成的复合物中的Epi却没有。这暗示着通过与铁形成络合物阻止了Epi与肾上腺素能受体的结合及其活化。Epi-Fe2 +复合物的促氧化活性可能代表了慢性应激与心血管疾病之间的联系。另一方面,不稳定的铁可以充当配体生物活性的调节剂。这种相互作用在与铁超负荷或缺乏有关的人类病理中可能很重要。Epi-Fe2 +复合物的促氧化活性可能代表了慢性应激与心血管疾病之间的联系。另一方面,不稳定的铁可以充当配体生物活性的调节剂。这种相互作用在与铁超负荷或缺乏有关的人类病理中可能很重要。Epi-Fe2 +复合物的促氧化活性可能代表了慢性应激与心血管疾病之间的联系。另一方面,不稳定的铁可以充当配体生物活性的调节剂。这种相互作用在与铁超负荷或缺乏有关的人类病理中可能很重要。
更新日期:2020-01-04
中文翻译:

铁与肾上腺素的结合促进铁的氧化并阻碍肾上腺素能受体的激活。
在响应压力而释放时,肾上腺素(Epi)可能与人体血浆中的不稳定铁池相互作用,从而可能产生重要的(病理)生理后果。我们已经显示Epi和Fe3 +在生理pH值下可建立稳定的1:1高纺丝双齿复合物,并且Epi在铁存在下不会发生降解。然而,Epi与更易溶的Fe2 +的相互作用以及铁对Epi的生物活性的影响仍然未知。在这里,我们表明Epi和Fe2 +生成无色络合物,在厌氧条件下稳定。在氧气存在下,Epi促进了Fe2 +的氧化和Epi-Fe3 +络合物的形成。循环伏安法显示Epi-Fe2 +络合物的中点电势非常低(相对于标准氢电极为-582 mV),这说明了Fe2 +的催化氧化。下一个,我们使用肾上腺素能受体的组成型表达在细胞培养中使用膜片钳研究了铁结合对Epi生物学性能的影响。单独的Epi引起外向电流的增加,而与Fe3 +形成的复合物中的Epi却没有。这暗示着通过与铁形成络合物阻止了Epi与肾上腺素能受体的结合及其活化。Epi-Fe2 +复合物的促氧化活性可能代表了慢性应激与心血管疾病之间的联系。另一方面,不稳定的铁可以充当配体生物活性的调节剂。这种相互作用在与铁超负荷或缺乏有关的人类病理中可能很重要。单独的Epi引起外向电流的增加,而与Fe3 +形成的复合物中的Epi却没有。这暗示着通过与铁形成络合物阻止了Epi与肾上腺素能受体的结合及其活化。Epi-Fe2 +复合物的促氧化活性可能代表了慢性应激与心血管疾病之间的联系。另一方面,不稳定的铁可以充当配体生物活性的调节剂。这种相互作用在与铁超负荷或缺乏有关的人类病理中可能很重要。单独的Epi引起外向电流的增加,而与Fe3 +形成的复合物中的Epi却没有。这暗示着通过与铁形成络合物阻止了Epi与肾上腺素能受体的结合及其活化。Epi-Fe2 +复合物的促氧化活性可能代表了慢性应激与心血管疾病之间的联系。另一方面,不稳定的铁可以充当配体生物活性的调节剂。这种相互作用在与铁超负荷或缺乏有关的人类病理中可能很重要。Epi-Fe2 +复合物的促氧化活性可能代表了慢性应激与心血管疾病之间的联系。另一方面,不稳定的铁可以充当配体生物活性的调节剂。这种相互作用在与铁超负荷或缺乏有关的人类病理中可能很重要。Epi-Fe2 +复合物的促氧化活性可能代表了慢性应激与心血管疾病之间的联系。另一方面,不稳定的铁可以充当配体生物活性的调节剂。这种相互作用在与铁超负荷或缺乏有关的人类病理中可能很重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号