当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanostructured degradable macroporous hydrogel scaffolds with controllable internal morphologies via reactive electrospinning.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.actbio.2019.12.038 Fei Xu 1 , Ian Gough 1 , Jonathan Dorogin 1 , Heather Sheardown 1 , Todd Hoare 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.actbio.2019.12.038 Fei Xu 1 , Ian Gough 1 , Jonathan Dorogin 1 , Heather Sheardown 1 , Todd Hoare 1
Affiliation
|
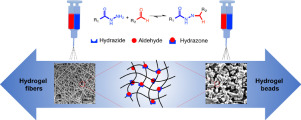
Creating micro/nanostructured hydrogels with tunable morphologies under cell-friendly processing conditions would enable rational engineering of hydrogel scaffolds for targeted biomedical applications. Herein, an all-aqueous single-step reactive electrospinning method is applied to prepare hydrogel networks with controlled morphologies on both the nanoscale and the microscale. Hydrazide and aldehyde-functionalized poly(oligo ethylene glycol methacrylate) (POEGMA) are co-spun from a double barrel syringe together with poly(ethylene oxide) (PEO) as an electrospinning aid. By varying the concentrations and molecular weights of PEO and/or POEGMA, various morphologies from pure fibers to beaded fibers to bead network morphologies with tunable bead sizes can be fabricated, all of which remain monolithically stable in water due to the dynamic covalent crosslinks formed within the gel structure. The rates and magnitudes of swelling, degradation, and mechanics of the resulting scaffolds can be tuned by independently controlling gel morphologies on the nanoscale (i.e. crosslink density within the gel) and the microscale (i.e. the network structure formed), with an atypical independence of swelling relative to the mechanics and degradation rate observed. Furthermore, the internal morphology of the networks is demonstrated to systematically alter both the cell responses within the scaffolds and the rate of protein release from the scaffolds, with small fibers showing optimal cell proliferation, bead networks exhibiting the slowest protein release kinetics and very high maintained cell viabilities post-electrospinning, and beaded fibers showing intermediate properties. STATEMENT OF SIGNIFICANCE: Controlling the internal structure of hydrogels is critical to successfully applying hydrogels in biomedical applications such as tissue engineering or cell/drug delivery. However, current techniques to fabricate hydrogel scaffolds typically require additives or gelation processes that are poorly compatible with cells and/or require multi-step processes. In this paper, we describe the fabrication of hydrogel scaffolds with tunable feature sizes (from nanometer to micrometer scale) and structures (from all fibers to bead/fiber mixtures to a new "bead network" morphology) using a reactive electrospinning strategy leveraging dynamic hydrazone crosslinking. We show single-step cell/protein loading and systematic control over cell proliferation and protein release kinetics by systematically manipulating the scaffold morphologies and feature sizes, allowing facile customization of scaffold properties for targeted applications.
中文翻译:

纳米结构可降解大孔水凝胶支架,可通过反应电纺丝控制内部形态。
在细胞友好的加工条件下创建具有可调整形态的微/纳米结构水凝胶将使合理设计水凝胶支架用于目标生物医学应用。在本文中,采用全水单步反应电纺丝方法来制备在纳米级和微米级上具有可控形态的水凝胶网络。酰肼和醛官能化的聚甲基丙烯酸低聚乙二醇酯(POEGMA)与双聚环氧乙烷(PEO)作为电纺助剂一起从双筒注射器中共纺而成。通过改变PEO和/或POEGMA的浓度和分子量,可以制造各种形态,从纯纤维到串珠纤维,再到具有可变珠大小的珠网络形态,由于在凝胶结构中形成的动态共价交联键,所有这些均在水中保持整体稳定。可以通过独立控制纳米级(即凝胶内的交联密度)和微米级(即形成的网络结构)的凝胶形态来调节所得支架的溶胀,降解和力学的速率和大小,其非典型独立性为相对于机械膨胀和观察到的降解率。此外,网络的内部形态被证明可以系统地改变支架内的细胞反应和从支架释放蛋白质的速率,其中小纤维显示出最佳的细胞增殖,珠状网络表现出最慢的蛋白质释放动力学,并且保持很高的维持率。静电纺丝后的细胞活力,以及具有中间性能的串珠纤维。意义声明:控制水凝胶的内部结构对于将水凝胶成功应用于生物医学应用(如组织工程或细胞/药物输送)至关重要。然而,当前制造水凝胶支架的技术通常需要与细胞相容性差的添加剂或胶凝工艺和/或需要多步工艺。在本文中,我们描述了利用动态利用反应静电纺丝策略制备具有可调特征尺寸(从纳米到微米级)和结构(从所有纤维到珠/纤维混合物到新的“珠网络”形态)的水凝胶支架的方法。交联。
更新日期:2020-01-04
中文翻译:

纳米结构可降解大孔水凝胶支架,可通过反应电纺丝控制内部形态。
在细胞友好的加工条件下创建具有可调整形态的微/纳米结构水凝胶将使合理设计水凝胶支架用于目标生物医学应用。在本文中,采用全水单步反应电纺丝方法来制备在纳米级和微米级上具有可控形态的水凝胶网络。酰肼和醛官能化的聚甲基丙烯酸低聚乙二醇酯(POEGMA)与双聚环氧乙烷(PEO)作为电纺助剂一起从双筒注射器中共纺而成。通过改变PEO和/或POEGMA的浓度和分子量,可以制造各种形态,从纯纤维到串珠纤维,再到具有可变珠大小的珠网络形态,由于在凝胶结构中形成的动态共价交联键,所有这些均在水中保持整体稳定。可以通过独立控制纳米级(即凝胶内的交联密度)和微米级(即形成的网络结构)的凝胶形态来调节所得支架的溶胀,降解和力学的速率和大小,其非典型独立性为相对于机械膨胀和观察到的降解率。此外,网络的内部形态被证明可以系统地改变支架内的细胞反应和从支架释放蛋白质的速率,其中小纤维显示出最佳的细胞增殖,珠状网络表现出最慢的蛋白质释放动力学,并且保持很高的维持率。静电纺丝后的细胞活力,以及具有中间性能的串珠纤维。意义声明:控制水凝胶的内部结构对于将水凝胶成功应用于生物医学应用(如组织工程或细胞/药物输送)至关重要。然而,当前制造水凝胶支架的技术通常需要与细胞相容性差的添加剂或胶凝工艺和/或需要多步工艺。在本文中,我们描述了利用动态利用反应静电纺丝策略制备具有可调特征尺寸(从纳米到微米级)和结构(从所有纤维到珠/纤维混合物到新的“珠网络”形态)的水凝胶支架的方法。交联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号