当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Pyrolysis of Poly(ethylene terephthalate) in the Presence of Metal Oxides for Aromatic Hydrocarbon Recovery Using Tandem μ-Reactor-GC/MS
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.energyfuels.9b02915 Shogo Kumagai 1 , Ryota Yamasaki 1 , Tomohito Kameda 1 , Yuko Saito 1 , Atsushi Watanabe 2 , Chuichi Watanabe 2 , Norio Teramae 2, 3 , Toshiaki Yoshioka 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.energyfuels.9b02915 Shogo Kumagai 1 , Ryota Yamasaki 1 , Tomohito Kameda 1 , Yuko Saito 1 , Atsushi Watanabe 2 , Chuichi Watanabe 2 , Norio Teramae 2, 3 , Toshiaki Yoshioka 1
Affiliation
|
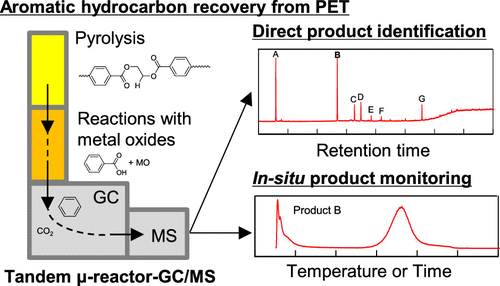
Poly(ethylene terephthalate) (PET) pyrolysis products and those produced from their subsequent catalytic reactions under various metal oxides (ZnO, MgO, TiO2, and ZrO2) were evaluated qualitatively and semiquantitatively using a tandem μ-reactor gas chromatography–mass spectrometry (TR-GC/MS) system. The catalytic reaction products were analyzed in situ to determine the duration and temperature dependence of their production. In the TR-GC/MS, a reactor with two-tier, independent heat sources was linked directly to a GC/MS device. PET pyrolysis was carried out at 450 °C, whereas the pyrolysis products were reacted in the presence of metal oxides at 700 °C. ZnO, which has a high base strength, promoted decarboxylation of the principal pyrolysis products of benzoic acid and terephthalic acid (TPA) selectively and at a low temperature. The proportion of oil components made up by benzene was up to 88.8 area%. On the other hand, MgO, TiO2, and ZrO2 have lower base strengths than ZnO. Hence, their capabilities for benzoic acid and TPA decarboxylation were low, and carboxylation using these oxides required temperatures 50–70 °C higher than that using ZnO. In summary, the present study found that benzene-rich aromatic hydrocarbons can be obtained by the catalytic pyrolysis of PET using various metal oxides and that the product composition depends on the acid–base properties of the metal oxides. These findings will help promote feedstock recycling to convert PET waste materials or mixed plastics that contain PET into raw chemical materials by pyrolysis.
中文翻译:

串联μ反应器GC / MS在金属氧化物存在下催化热解聚对苯二甲酸乙二醇酯以回收芳烃
聚对苯二甲酸乙二醇酯(PET)热解产物及其在各种金属氧化物(ZnO,MgO,TiO 2和ZrO 2下)的后续催化反应产生的产物)是使用串联μ反应器气相色谱-质谱(TR-GC / MS)系统定性和半定量评估的。对催化反应产物进行原位分析以确定其生产的持续时间和温度依赖性。在TR-GC / MS中,具有两层独立热源的反应器直接连接到GC / MS设备。PET热解在450℃下进行,而热解产物在金属氧化物存在下在700℃下反应。具有高碱强度的ZnO在低温下选择性地促进了苯甲酸和对苯二甲酸(TPA)的主要热解产物的脱羧。苯所占的石油成分比例达到了88.8%。另一方面,MgO,TiO 2和ZrO 2具有比ZnO低的基本强度。因此,它们的苯甲酸和TPA脱羧能力很低,使用这些氧化物进行羧化所需的温度要比使用ZnO时高50-70°C。总而言之,本研究发现,可以使用各种金属氧化物通过PET的催化热解来获得富含苯的芳烃,并且产物的组成取决于金属氧化物的酸碱性质。这些发现将有助于促进原料回收,以通过热解将PET废料或包含PET的混合塑料转化为化学原料。
更新日期:2020-01-16
中文翻译:

串联μ反应器GC / MS在金属氧化物存在下催化热解聚对苯二甲酸乙二醇酯以回收芳烃
聚对苯二甲酸乙二醇酯(PET)热解产物及其在各种金属氧化物(ZnO,MgO,TiO 2和ZrO 2下)的后续催化反应产生的产物)是使用串联μ反应器气相色谱-质谱(TR-GC / MS)系统定性和半定量评估的。对催化反应产物进行原位分析以确定其生产的持续时间和温度依赖性。在TR-GC / MS中,具有两层独立热源的反应器直接连接到GC / MS设备。PET热解在450℃下进行,而热解产物在金属氧化物存在下在700℃下反应。具有高碱强度的ZnO在低温下选择性地促进了苯甲酸和对苯二甲酸(TPA)的主要热解产物的脱羧。苯所占的石油成分比例达到了88.8%。另一方面,MgO,TiO 2和ZrO 2具有比ZnO低的基本强度。因此,它们的苯甲酸和TPA脱羧能力很低,使用这些氧化物进行羧化所需的温度要比使用ZnO时高50-70°C。总而言之,本研究发现,可以使用各种金属氧化物通过PET的催化热解来获得富含苯的芳烃,并且产物的组成取决于金属氧化物的酸碱性质。这些发现将有助于促进原料回收,以通过热解将PET废料或包含PET的混合塑料转化为化学原料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号