当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilization Mechanism for a Nonfibrillar Amyloid β Oligomer Based on Formation of a Hydrophobic Core Determined by Dissipative Particle Dynamics.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-01-15 , DOI: 10.1021/acschemneuro.9b00602 Ryoko Kawai 1 , Shuntaro Chiba 2 , Koji Okuwaki 3 , Ryo Kanada 4 , Hideo Doi 5 , Masahiro Ono 6 , Yuji Mochizuki 3, 7 , Yasushi Okuno 1, 2, 4
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-01-15 , DOI: 10.1021/acschemneuro.9b00602 Ryoko Kawai 1 , Shuntaro Chiba 2 , Koji Okuwaki 3 , Ryo Kanada 4 , Hideo Doi 5 , Masahiro Ono 6 , Yuji Mochizuki 3, 7 , Yasushi Okuno 1, 2, 4
Affiliation
|
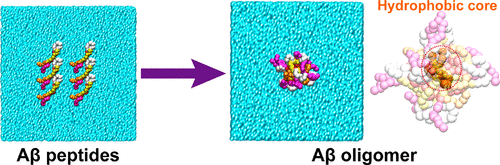
Neurotoxicity caused by nonfibrillar amyloid β (Aβ) oligomers in the brain is suggested to be associated with the onset of Alzheimer's disease (AD). Elucidating the structural features of Aβ oligomers is critical for promoting drug discovery research for AD. One of the Aβ oligomers, known as Aβ*56, is a dodecamer that impairs memory when injected into healthy rats, suggesting that Aβ*56 may contribute to cognitive deficits in AD patients. Another dodecamer structure, formed by 20-residue peptide segments derived from the Aβ peptide (Aβ17-36), has been revealed by X-ray crystallography. The structure of the Aβ17-36 dodecamer is composed of trimer units and shows the oligomer antibody A11 reactivity, which are characteristic of Aβ*56, indicating that Aβ*56 and the Aβ17-36 dodecamer share a similar structure. However, the structure of the C-terminal regions (Aβ37-42) remains unclear. The C-terminal region, which is abundant in hydrophobic residues, is thought to play a key role in stabilizing the oligomer structure by forming a hydrophobic core. In this study, we employed dissipative particle dynamics, a coarse-grained simulation method with soft core potentials, utilizing the crystal structure information to unravel Aβ dodecamer structures with C-terminal regions. The simulation results were validated by the reported experimental data. Hence, an analysis of the simulation results can provide structural insights into Aβ oligomers. Our simulations revealed the stabilization mechanism of the dodecamer structure at the molecular level. We showed that C-terminal regions spontaneously form a hydrophobic core in the central cavity, contributing to stabilizing the dodecamer structure. Furthermore, four consecutive hydrophobic residues in the C-terminal region (i.e., Val39-Ala42) are important for core formation.
中文翻译:

基于由耗散粒子动力学确定的疏水核的形成,非原纤维淀粉样β低聚物的稳定机理。
脑中非原纤维淀粉样蛋白β(Aβ)低聚物引起的神经毒性被认为与阿尔茨海默氏病(AD)的发作有关。阐明Aβ寡聚体的结构特征对于促进AD的药物发现研究至关重要。一种Aβ低聚物,称为Aβ* 56,是一种十二聚体,在注入健康大鼠后会损害记忆,表明Aβ* 56可能导致AD患者的认知功能障碍。通过X射线晶体学揭示了由源自Aβ肽的20个残基的肽区段形成的另一十二聚体结构(Aβ17-36)。Aβ17-36十二聚体的结构由三聚体单元构成,并且显示出寡聚抗体A11反应性,其是Aβ* 56的特征,表明Aβ* 56和Aβ17-36十二聚体具有相似的结构。然而,C末端区域(Aβ37-42)的结构仍不清楚。认为在疏水残基中丰富的C末端区域在通过形成疏水核来稳定低聚物结构中起关键作用。在这项研究中,我们采用了耗散粒子动力学,一种具有软核势的粗粒度模拟方法,利用晶体结构信息来解开具有C端区域的Aβ十二聚体结构。通过实验数据验证了仿真结果。因此,对模拟结果的分析可以为Aβ低聚物提供结构上的见识。我们的模拟揭示了十二聚体结构在分子水平上的稳定机理。我们发现C端区域在中央空腔中自发形成疏水核,有助于稳定十二头肌结构。此外,在C端区域中的四个连续的疏水残基(即Val39-Ala42)对于核心形成很重要。
更新日期:2020-01-16
中文翻译:

基于由耗散粒子动力学确定的疏水核的形成,非原纤维淀粉样β低聚物的稳定机理。
脑中非原纤维淀粉样蛋白β(Aβ)低聚物引起的神经毒性被认为与阿尔茨海默氏病(AD)的发作有关。阐明Aβ寡聚体的结构特征对于促进AD的药物发现研究至关重要。一种Aβ低聚物,称为Aβ* 56,是一种十二聚体,在注入健康大鼠后会损害记忆,表明Aβ* 56可能导致AD患者的认知功能障碍。通过X射线晶体学揭示了由源自Aβ肽的20个残基的肽区段形成的另一十二聚体结构(Aβ17-36)。Aβ17-36十二聚体的结构由三聚体单元构成,并且显示出寡聚抗体A11反应性,其是Aβ* 56的特征,表明Aβ* 56和Aβ17-36十二聚体具有相似的结构。然而,C末端区域(Aβ37-42)的结构仍不清楚。认为在疏水残基中丰富的C末端区域在通过形成疏水核来稳定低聚物结构中起关键作用。在这项研究中,我们采用了耗散粒子动力学,一种具有软核势的粗粒度模拟方法,利用晶体结构信息来解开具有C端区域的Aβ十二聚体结构。通过实验数据验证了仿真结果。因此,对模拟结果的分析可以为Aβ低聚物提供结构上的见识。我们的模拟揭示了十二聚体结构在分子水平上的稳定机理。我们发现C端区域在中央空腔中自发形成疏水核,有助于稳定十二头肌结构。此外,在C端区域中的四个连续的疏水残基(即Val39-Ala42)对于核心形成很重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号