当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Magnetic Mesoporous Calcium Carbonate-Based Nanocomposites for the Removal of Toxic Pb(II) and Cd(II) Ions from Water
ACS Applied Nano Materials ( IF 5.9 ) Pub Date : 2020-01-21 , DOI: 10.1021/acsanm.9b02036 Penglei Wang 1 , Tingting Shen 2, 3 , Xinyong Li 1 , Yuanyuan Tang 2 , Yongjie Li 3
ACS Applied Nano Materials ( IF 5.9 ) Pub Date : 2020-01-21 , DOI: 10.1021/acsanm.9b02036 Penglei Wang 1 , Tingting Shen 2, 3 , Xinyong Li 1 , Yuanyuan Tang 2 , Yongjie Li 3
Affiliation
|
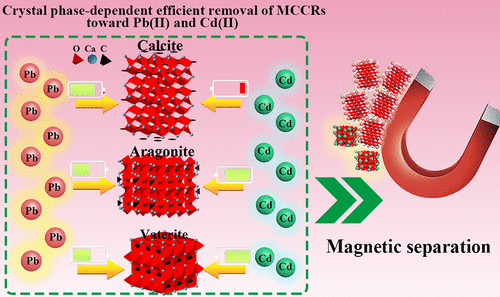
Calcium carbonate (CaCO3)-based nanoadsorbents have gained widespread attention due to their superiority in removing toxic heavy metal ions. Considering that the inherent structure of the material determines its performance, it is important to fully understand the effect of different crystals of CaCO3 on the removal of heavy metals. On the other hand, the difficult separation of nanoadsorbents during in situ remediation may cause the potential risk of secondary pollution by the remaining adsorbents. Herein, the magnetic calcium carbonate adsorbents with nanostructure (MCCR-X, X represent the annealing temperature) were fabricated with a tunable crystal structure of CaCO3 by adjusting the annealing temperature. Results from batch experiments revealed the crystal structure (CaCO3)-dependent adsorption performance of MCCRs toward Pb(II) and Cd(II). MCCR-350 with an aragonite phase showed the outstanding adsorption toward both Pb(II) and Cd(II) with maximum adsorption capacity of 1179 and 821 mg/g, and MCCR-RAW with a vaterite/aragonite phase also has similar adsorption properties. However, MCCR-550 with the calcite phase displayed a superhigh adsorption toward Pb(II) only but very weak adsorption toward Cd(II) with a capacity of 1350 and 30 mg/g in the initial concentration of 300 mg/g. The findings indicated that the efficiently tunable removal of the heavy metal ion Pb(II) or Cd(II) by MCCRs can be realized by regulating the crystal phase of CaCO3. X-ray photoelectron spectroscopy technology (XPS) was employed to clarify the possible mechanism of the crystal phase of CaCO3-dependent adsorption ability for Pb(II)/Cd(II) removal by MCCRs. This work provides an understanding of the crystal-structure-related adsorption properties of CaCO3 and also gives guidance for designing a CaCO3-based adsorbent for removal/recovery of heavy metal ions.
中文翻译:

磁性介孔碳酸钙基纳米复合材料从水中去除有毒的Pb(II)和Cd(II)离子
碳酸钙(CaCO 3)基纳米吸附剂因其在去除有毒重金属离子方面的优越性而受到广泛关注。考虑到材料的固有结构决定了其性能,重要的是要充分了解CaCO 3的不同晶体对重金属去除的影响。另一方面,纳米吸附剂在原位修复过程中难以分离可能会导致残留吸附剂造成二次污染的潜在风险。在此,制备了具有纳米结构的磁性碳酸钙吸附剂(MCCR- X,X表示退火温度),其具有可调的CaCO 3晶体结构。通过调节退火温度。批处理实验的结果表明,MCCR对Pb(II)和Cd(II)的晶体结构(CaCO 3)依赖性吸附性能。具有文石相的MCCR-350对Pb(II)和Cd(II)均表现出出色的吸附性能,最大吸附容量为1179和821 mg / g,而具有球ate石/文石相的MCCR-RAW也具有相似的吸附性能。然而,具有方解石相的MCCR-550仅对Pb(II)表现出超高吸附,而对Cd(II)的吸附却非常弱,在初始浓度为300 mg / g时容量为1350和30 mg / g。这些发现表明,通过调节CaCO 3的晶相,可以实现MCCR有效地可调谐地去除重金属离子Pb(II)或Cd(II)。。采用X射线光电子能谱技术(XPS)阐明了CaCR 3依赖的晶相通过MCCR去除Pb(II)/ Cd(II)的吸附能力的可能机理。这项工作提供了对CaCO 3的晶体结构相关吸附特性的理解,并为设计用于去除/回收重金属离子的基于CaCO 3的吸附剂提供了指导。
更新日期:2020-01-22
中文翻译:

磁性介孔碳酸钙基纳米复合材料从水中去除有毒的Pb(II)和Cd(II)离子
碳酸钙(CaCO 3)基纳米吸附剂因其在去除有毒重金属离子方面的优越性而受到广泛关注。考虑到材料的固有结构决定了其性能,重要的是要充分了解CaCO 3的不同晶体对重金属去除的影响。另一方面,纳米吸附剂在原位修复过程中难以分离可能会导致残留吸附剂造成二次污染的潜在风险。在此,制备了具有纳米结构的磁性碳酸钙吸附剂(MCCR- X,X表示退火温度),其具有可调的CaCO 3晶体结构。通过调节退火温度。批处理实验的结果表明,MCCR对Pb(II)和Cd(II)的晶体结构(CaCO 3)依赖性吸附性能。具有文石相的MCCR-350对Pb(II)和Cd(II)均表现出出色的吸附性能,最大吸附容量为1179和821 mg / g,而具有球ate石/文石相的MCCR-RAW也具有相似的吸附性能。然而,具有方解石相的MCCR-550仅对Pb(II)表现出超高吸附,而对Cd(II)的吸附却非常弱,在初始浓度为300 mg / g时容量为1350和30 mg / g。这些发现表明,通过调节CaCO 3的晶相,可以实现MCCR有效地可调谐地去除重金属离子Pb(II)或Cd(II)。。采用X射线光电子能谱技术(XPS)阐明了CaCR 3依赖的晶相通过MCCR去除Pb(II)/ Cd(II)的吸附能力的可能机理。这项工作提供了对CaCO 3的晶体结构相关吸附特性的理解,并为设计用于去除/回收重金属离子的基于CaCO 3的吸附剂提供了指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号