当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fabrication of New Belousov-Zhabotinsky Micro-Oscillators on the Basis of Silica Gel Beads.
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.jpca.9b09127 Ilya L Mallphanov 1 , Vladimir K Vanag 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.jpca.9b09127 Ilya L Mallphanov 1 , Vladimir K Vanag 1
Affiliation
|
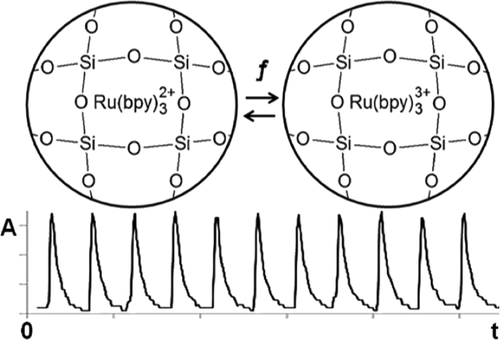
We have fabricated and examined silica gel beads loaded with a catalyst of the Belousov-Zhabotinsky (BZ) reaction, tris(2,2'-bipyridyl)ruthenium(II) chloride, Ru(bpy)3Cl2, the BZ beads. The abilities of silica gel and the widely used ion-exchange resin (Dowex 50WX2) BZ beads to oscillate in a catalyst-free BZ solution are compared. The period of the BZ oscillations increases with an increase in the diameter (40-250 μm) of both types of the BZ beads. The ability of the ion-exchange resin beads to hold catalyst molecules significantly decreases with a decrease in pH, while this value is less dependent on pH for the silica gel BZ beads. The diffusive coupling of two BZ beads separated by either aqueous or oil gaps is studied as well. For the aqueous gap less than 100 μm, the BZ beads of both types demonstrate in-phase synchronization. For the oil phase, the Dowex BZ beads are unable to oscillate for a long enough time and therefore cannot be synchronized, while the silica gel BZ beads are able to oscillate for several hours and demonstrate anti-phase synchronization for gaps less than 40 μm.
中文翻译:

基于硅胶微珠的新型Belousov-Zhabotinsky微振荡器的制造。
我们已经制备并检查了硅胶珠,其中负载有Belousov-Zhabotinsky(BZ)反应的催化剂,氯化三(2,2'-联吡啶基)钌(II),Ru(bpy)3Cl2,BZ珠。比较了硅胶和广泛使用的离子交换树脂(Dowex 50WX2)BZ珠在无催化剂的BZ溶液中振荡的能力。两种类型的BZ磁珠的直径(40-250μm)越大,BZ振荡的周期就越大。离子交换树脂珠粒保持催化剂分子的能力会随着pH的降低而显着降低,而该值对硅胶BZ珠粒的pH依赖性较小。还研究了两个BZ珠的扩散耦合,它们被水或油隙分开。对于小于100μm的水隙,两种类型的BZ珠都显示出同相同步。
更新日期:2020-01-04
中文翻译:

基于硅胶微珠的新型Belousov-Zhabotinsky微振荡器的制造。
我们已经制备并检查了硅胶珠,其中负载有Belousov-Zhabotinsky(BZ)反应的催化剂,氯化三(2,2'-联吡啶基)钌(II),Ru(bpy)3Cl2,BZ珠。比较了硅胶和广泛使用的离子交换树脂(Dowex 50WX2)BZ珠在无催化剂的BZ溶液中振荡的能力。两种类型的BZ磁珠的直径(40-250μm)越大,BZ振荡的周期就越大。离子交换树脂珠粒保持催化剂分子的能力会随着pH的降低而显着降低,而该值对硅胶BZ珠粒的pH依赖性较小。还研究了两个BZ珠的扩散耦合,它们被水或油隙分开。对于小于100μm的水隙,两种类型的BZ珠都显示出同相同步。



























 京公网安备 11010802027423号
京公网安备 11010802027423号