当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Label-Free Analysis of Multivalent Protein Binding Using Bioresponsive Nanogels and Surface Plasmon Resonance (SPR).
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-01-14 , DOI: 10.1021/acsami.9b17328 Hae Min Yang 1 , Jie Ying Teoh 1 , Guk Hee Yim 1 , Yongdoo Park 2 , Young Gyu Kim 1, 3 , Jongseong Kim 2 , Dongwon Yoo 1, 3, 4
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-01-14 , DOI: 10.1021/acsami.9b17328 Hae Min Yang 1 , Jie Ying Teoh 1 , Guk Hee Yim 1 , Yongdoo Park 2 , Young Gyu Kim 1, 3 , Jongseong Kim 2 , Dongwon Yoo 1, 3, 4
Affiliation
|
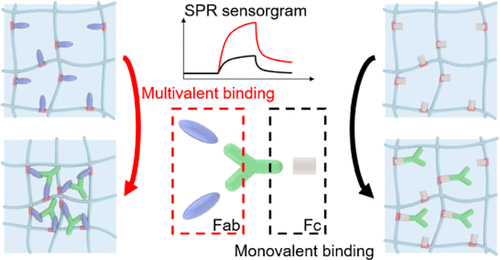
Precise identification of protein-protein interactions is required to improve our understanding of biochemical pathways for biology and medicine. In physiology, how proteins interact with other proteins or small molecules is crucial for maintaining biological functions. For instance, multivalent protein binding (MPB), in which a ligand concurrently interacts with two or more receptors, plays a key role in regulating complex but accurate biological functions, and its interference is related to many diseases. Therefore, determining MPB and its kinetics has long been sought, which currently requires complicated procedures and instruments to distinguish multivalent binding from monovalent binding. Here, we show a method for quickly evaluating the MPB over monovalent binding and its kinetic parameters in a label-free manner. Engaging pNIPAm-co-AAc nanogels with MPB-capable moieties (e.g., PD-1 antigen and biocytin) permits a surface plasmon resonance (SPR) instrument to evaluate the MPB events by amplifying signals from the specific target molecules. Using our MPB-based method, PD-1 antibody that forms a type of MPB by complexing with two PD-1 proteins, which are currently used for cancer immunotherapy, is detectable down to a level of 10 nM. In addition, small multivalent cations (e.g., Ca2+, Fe2+, and Fe3+) are distinguishably measurable over monovalent cations (e.g., Na+ and K+) with the pNIPAm-co-AAc nanogels.
中文翻译:

使用生物响应性纳米凝胶和表面等离振子共振(SPR)的多价蛋白结合的无标记分析。
需要精确鉴定蛋白质间相互作用,以增进我们对生物学和医学生化途径的了解。在生理学中,蛋白质如何与其他蛋白质或小分子相互作用是维持生物学功能的关键。例如,配体同时与两个或多个受体相互作用的多价蛋白结合(MPB)在调节复杂但准确的生物学功能中起关键作用,并且其干扰与许多疾病有关。因此,长期以来一直寻求确定MPB及其动力学,这需要复杂的程序和仪器来区分多价结合与单价结合。在这里,我们展示了一种以无标记方式快速评估MPB单价结合及其动力学参数的方法。使pNIPAm-co-AAc纳米凝胶与具有MPB功能的部分(例如PD-1抗原和生物胞素)结合,可以使表面等离振子共振(SPR)仪器通过放大来自特定靶分子的信号来评估MPB事件。使用我们基于MPB的方法,通过与目前用于癌症免疫治疗的两种PD-1蛋白复合形成一种MPB的PD-1抗体可检测到10 nM的水平。此外,使用pNIPAm-co-AAc纳米凝胶,可以比一价阳离子(例如Na +和K +)更好地测量小的多价阳离子(例如Ca2 +,Fe2 +和Fe3 +)。通过与目前用于癌症免疫治疗的两种PD-1蛋白复合形成一种MPB的PD-1抗体可检测到低至10 nM的水平。此外,使用pNIPAm-co-AAc纳米凝胶,可以比一价阳离子(例如Na +和K +)更好地测量小的多价阳离子(例如Ca2 +,Fe2 +和Fe3 +)。通过与目前用于癌症免疫治疗的两种PD-1蛋白复合形成一种MPB的PD-1抗体可检测到低至10 nM的水平。此外,使用pNIPAm-co-AAc纳米凝胶,可以比一价阳离子(例如Na +和K +)更好地测量小的多价阳离子(例如Ca2 +,Fe2 +和Fe3 +)。
更新日期:2020-01-15
中文翻译:

使用生物响应性纳米凝胶和表面等离振子共振(SPR)的多价蛋白结合的无标记分析。
需要精确鉴定蛋白质间相互作用,以增进我们对生物学和医学生化途径的了解。在生理学中,蛋白质如何与其他蛋白质或小分子相互作用是维持生物学功能的关键。例如,配体同时与两个或多个受体相互作用的多价蛋白结合(MPB)在调节复杂但准确的生物学功能中起关键作用,并且其干扰与许多疾病有关。因此,长期以来一直寻求确定MPB及其动力学,这需要复杂的程序和仪器来区分多价结合与单价结合。在这里,我们展示了一种以无标记方式快速评估MPB单价结合及其动力学参数的方法。使pNIPAm-co-AAc纳米凝胶与具有MPB功能的部分(例如PD-1抗原和生物胞素)结合,可以使表面等离振子共振(SPR)仪器通过放大来自特定靶分子的信号来评估MPB事件。使用我们基于MPB的方法,通过与目前用于癌症免疫治疗的两种PD-1蛋白复合形成一种MPB的PD-1抗体可检测到10 nM的水平。此外,使用pNIPAm-co-AAc纳米凝胶,可以比一价阳离子(例如Na +和K +)更好地测量小的多价阳离子(例如Ca2 +,Fe2 +和Fe3 +)。通过与目前用于癌症免疫治疗的两种PD-1蛋白复合形成一种MPB的PD-1抗体可检测到低至10 nM的水平。此外,使用pNIPAm-co-AAc纳米凝胶,可以比一价阳离子(例如Na +和K +)更好地测量小的多价阳离子(例如Ca2 +,Fe2 +和Fe3 +)。通过与目前用于癌症免疫治疗的两种PD-1蛋白复合形成一种MPB的PD-1抗体可检测到低至10 nM的水平。此外,使用pNIPAm-co-AAc纳米凝胶,可以比一价阳离子(例如Na +和K +)更好地测量小的多价阳离子(例如Ca2 +,Fe2 +和Fe3 +)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号