当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The First Step in Catalysis of the Radical S-Adenosylmethionine Methylthiotransferase MiaB Yields an Intermediate with a [3Fe-4S]0-like Auxiliary Cluster
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-01-03 , DOI: 10.1021/jacs.9b11093 Bo Zhang , Arthur J. Arcinas , Matthew I. Radle , Alexey Silakov , Squire J. Booker , Carsten Krebs
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-01-03 , DOI: 10.1021/jacs.9b11093 Bo Zhang , Arthur J. Arcinas , Matthew I. Radle , Alexey Silakov , Squire J. Booker , Carsten Krebs
|
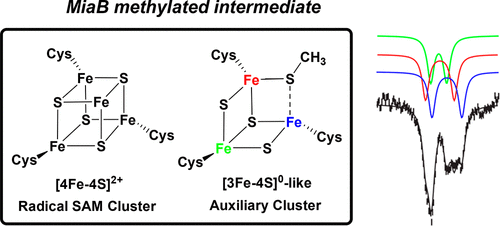
The enzyme MiaB catalyzes the attachment of a methylthio (-SCH3) group at the C2 position of N6-(isopentenyl)adenosine (i6A) in the final step of the biosynthesis of the hypermodified tRNA nucleotide 2-methythio-N6-(isopentenyl)adenosine (ms2i6A). MiaB belongs to the expanding subgroup of enzymes of the radical S-adenosylmethionine (SAM) superfamily that harbor one or more auxiliary [4Fe-4S] clusters in addition to the [4Fe-4S] cluster that all family members require for the reductive cleavage of SAM to afford the common 5'-deoxyadenosyl 5'-radical (5'-dA•) intermediate. While the role of the radical SAM cluster in generating the 5'-dA• is well understood, the detailed role of the auxiliary cluster, which is essential for MiaB catalysis, remains unclear. It has been proposed that the auxiliary cluster may either serve as a coordination site for exogenously derived sulfur destined for attachment to the substrate, or that the cluster itself provides the sulfur atom and is sacrificed during turnover. In this work, we report spectroscopic and biochemical evidence that the auxiliary [4Fe-4S]2+ cluster in Bacteroides thetaiotaomicron (Bt) MiaB is converted to a [3Fe-4S]0-like cluster during the methylation step of catalysis. Mössbauer characterization of the MiaB [3Fe-4S]0-like cluster revealed unusual spectroscopic properties compared to those of other well-characterized cuboidal [3Fe-4S]0 clusters. Specifically, the Fe sites of the mixed-valent moiety do not have identical Mössbauer parameters. Our results support a mechanism where the auxiliary [4Fe-4S] cluster is the direct sulfur source during catalysis.
中文翻译:

自由基 S-腺苷甲硫氨酸甲硫基转移酶 MiaB 催化的第一步产生具有 [3Fe-4S]0 样辅助簇的中间体
在超修饰 tRNA 核苷酸 2-甲硫基-N6-(异戊烯基) 腺苷生物合成的最后一步中,MiaB 酶催化甲硫基 (-SCH3) 基团附着在 N6-(异戊烯基) 腺苷 (i6A) 的 C2 位上(ms2i6A)。MiaB 属于自由基 S-腺苷甲硫氨酸 (SAM) 超家族的扩展酶亚组,除了所有家族成员还原裂解所需的 [4Fe-4S] 簇之外,还包含一个或多个辅助 [4Fe-4S] 簇。 SAM 提供常见的 5'-脱氧腺苷 5'-自由基 (5'-dA•) 中间体。虽然自由基 SAM 簇在生成 5'-dA• 中的作用已广为人知,但对于 MiaB 催化至关重要的辅助簇的详细作用仍不清楚。有人提出,辅助簇可以作为外源硫的配位位点,用于附着在底物上,或者簇本身提供硫原子并在周转过程中被牺牲。在这项工作中,我们报告了光谱和生化证据,表明拟杆菌 (Bt) MiaB 中的辅助 [4Fe-4S]2+ 簇在催化甲基化步骤中转化为 [3Fe-4S]0 样簇。与其他充分表征的立方体 [3Fe-4S]0 星团相比,MiaB [3Fe-4S]0 类星团的穆斯堡尔表征揭示了不寻常的光谱特性。具体来说,混合价部分的 Fe 位点不具有相同的穆斯堡尔参数。我们的结果支持了一种机制,其中辅助[4Fe-4S]簇是催化过程中的直接硫源。
更新日期:2020-01-03
中文翻译:

自由基 S-腺苷甲硫氨酸甲硫基转移酶 MiaB 催化的第一步产生具有 [3Fe-4S]0 样辅助簇的中间体
在超修饰 tRNA 核苷酸 2-甲硫基-N6-(异戊烯基) 腺苷生物合成的最后一步中,MiaB 酶催化甲硫基 (-SCH3) 基团附着在 N6-(异戊烯基) 腺苷 (i6A) 的 C2 位上(ms2i6A)。MiaB 属于自由基 S-腺苷甲硫氨酸 (SAM) 超家族的扩展酶亚组,除了所有家族成员还原裂解所需的 [4Fe-4S] 簇之外,还包含一个或多个辅助 [4Fe-4S] 簇。 SAM 提供常见的 5'-脱氧腺苷 5'-自由基 (5'-dA•) 中间体。虽然自由基 SAM 簇在生成 5'-dA• 中的作用已广为人知,但对于 MiaB 催化至关重要的辅助簇的详细作用仍不清楚。有人提出,辅助簇可以作为外源硫的配位位点,用于附着在底物上,或者簇本身提供硫原子并在周转过程中被牺牲。在这项工作中,我们报告了光谱和生化证据,表明拟杆菌 (Bt) MiaB 中的辅助 [4Fe-4S]2+ 簇在催化甲基化步骤中转化为 [3Fe-4S]0 样簇。与其他充分表征的立方体 [3Fe-4S]0 星团相比,MiaB [3Fe-4S]0 类星团的穆斯堡尔表征揭示了不寻常的光谱特性。具体来说,混合价部分的 Fe 位点不具有相同的穆斯堡尔参数。我们的结果支持了一种机制,其中辅助[4Fe-4S]簇是催化过程中的直接硫源。



























 京公网安备 11010802027423号
京公网安备 11010802027423号