当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
“Outlaw” Dipole-Bound Anions of Intra-Molecular Complexes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-03 , DOI: 10.1021/jacs.9b11694 Valery F Sidorkin 1 , Elena F Belogolova 1 , Evgeniya P Doronina 1 , Gaoxiang Liu 2 , Sandra M Ciborowski 2 , Kit H Bowen 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-03 , DOI: 10.1021/jacs.9b11694 Valery F Sidorkin 1 , Elena F Belogolova 1 , Evgeniya P Doronina 1 , Gaoxiang Liu 2 , Sandra M Ciborowski 2 , Kit H Bowen 2
Affiliation
|
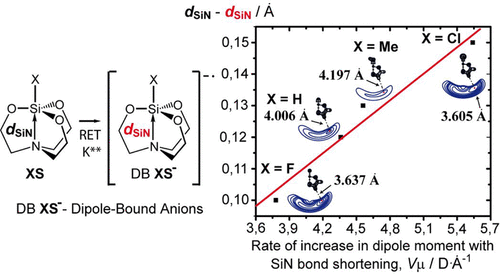
Using the example of silatranes XSi(OCH2CH2)3N (X = Me, H, F, Cl), XS, it was found that the effect of the dipole-bound (DB) electron on the cage intramolecular complexes does not fit into the standard views. Upon the transition from XS to the DB anions XS-, the unusual shortening of the internuclear Si→N distance is always observed. For X = Cl it is equal to 0.15 Å, which is a record length for all DB anions known from the literature. The formation of DB anions with the cage structure has principal features. It is controlled not only by the "critical" value of the dipole moment (μ > 2.5 D), but also by a geometric factor, such as the degree of pyramidality of the N(CH2)3 moiety - the positive end of the molecular dipole of XS. It was a surprise that the effect of the substituent X on the extent of the structural rearrangement in the process XS → XS- cannot be explained using the values of the electron detachment energy of XS- or the initial strength of the coordination Si←N bond in XS. The unique sensitivity of the silatrane geometry to the addition of an excess electron is governed by the rate of increase of their dipole moment with the shortening of the dative Si←N contact. The conclusions drawn are supported by the high-accuracy CCSD and CCSD(T) calculations and the experimental (RET-PES) data. There is no real reason to doubt that the peculiarities of the formation of DB anions of XS- can also be characteristic of many hundreds of their structural analogues XM(YCH2CH2)3N (M = Si, Ge, Sn, Pb, Ti, Al, Cr, Fe, Ni..; Y = O, NR, CH2, S) - ,i.e., substituted 5-azabicyclo[3.3.3]undecans.
中文翻译:

分子内复合物的“非法”偶极结合阴离子
以 silatranes XSi(OCH2CH2)3N (X = Me, H, F, Cl), XS 为例,发现偶极结合 (DB) 电子对笼式分子内复合物的影响不符合标准意见。在从 XS 过渡到 DB 阴离子 XS- 时,总是观察到核间 Si→N 距离的异常缩短。对于 X = Cl,它等于 0.15 Å,这是文献中已知的所有 DB 阴离子的记录长度。具有笼状结构的 DB 阴离子的形成具有主要特征。它不仅受偶极矩的“临界”值 (μ > 2.5 D) 的控制,还受几何因素的控制,例如 N(CH2)3 部分的金字塔度 - 分子的正端XS 的偶极子。令人惊讶的是,XS → XS- 过程中取代基 X 对结构重排程度的影响无法使用 XS- 的电子脱离能值或配位 Si←N 键的初始强度来解释在 XS。silatrane 几何形状对添加过量电子的独特敏感性由它们的偶极矩随着配价 Si←N 接触的缩短而增加的速率控制。得出的结论得到了高精度 CCSD 和 CCSD(T) 计算以及实验 (RET-PES) 数据的支持。没有真正的理由怀疑 XS- 的 DB 阴离子形成的特性也可以是其数百个结构类似物 XM(YCH2CH2)3N (M = Si、Ge、Sn、Pb、Ti、Al、 Cr, Fe, Ni..; Y = O, NR, CH2, S) - ,即,
更新日期:2020-01-03
中文翻译:

分子内复合物的“非法”偶极结合阴离子
以 silatranes XSi(OCH2CH2)3N (X = Me, H, F, Cl), XS 为例,发现偶极结合 (DB) 电子对笼式分子内复合物的影响不符合标准意见。在从 XS 过渡到 DB 阴离子 XS- 时,总是观察到核间 Si→N 距离的异常缩短。对于 X = Cl,它等于 0.15 Å,这是文献中已知的所有 DB 阴离子的记录长度。具有笼状结构的 DB 阴离子的形成具有主要特征。它不仅受偶极矩的“临界”值 (μ > 2.5 D) 的控制,还受几何因素的控制,例如 N(CH2)3 部分的金字塔度 - 分子的正端XS 的偶极子。令人惊讶的是,XS → XS- 过程中取代基 X 对结构重排程度的影响无法使用 XS- 的电子脱离能值或配位 Si←N 键的初始强度来解释在 XS。silatrane 几何形状对添加过量电子的独特敏感性由它们的偶极矩随着配价 Si←N 接触的缩短而增加的速率控制。得出的结论得到了高精度 CCSD 和 CCSD(T) 计算以及实验 (RET-PES) 数据的支持。没有真正的理由怀疑 XS- 的 DB 阴离子形成的特性也可以是其数百个结构类似物 XM(YCH2CH2)3N (M = Si、Ge、Sn、Pb、Ti、Al、 Cr, Fe, Ni..; Y = O, NR, CH2, S) - ,即,











































 京公网安备 11010802027423号
京公网安备 11010802027423号