当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvation structure and molecular interactions of ibuprofen with ethanol and water: A theoretical study
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.fluid.2019.112454 Mao Zhang , Yiping Huang , Dule Hao , Yuanhui Ji , Defang Ouyang
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.fluid.2019.112454 Mao Zhang , Yiping Huang , Dule Hao , Yuanhui Ji , Defang Ouyang
|
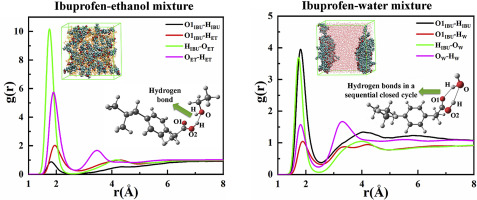
Abstract Molecular dynamics (MD) simulations were applied to systematically investigate the interactions and solvation structures of ibuprofen with ethanol or water at the temperature ranging from 298.15 K to 333.15 K. The results demonstrated that ibuprofen molecules mainly acted as HB donors in ethanol while in water as both HB donors and HB acceptors by establishing two HBs in a sequential closed cycle with water molecules. It was evident that the strength of hydrogen bonds (HBs) attenuated gradually in following orders: ibuprofen-ethanol > ethanol-ethanol > ibuprofen–ibuprofen (in ibuprofen-ethanol mixtures), ibuprofen-ibuprofen > ibuprofen-water > water-water (in ibuprofen-water mixtures). HBs weakened with increasing temperature, while the HB structure kept almost unchanged. A great amount of HBs in water molecules hindered the introduction of ibuprofen. Additionally, the electrostatic and van der Waals interactions, as well as the solvation structures of ibuprofen with both ethanol and water were nearly unaffected by temperature.
中文翻译:

布洛芬与乙醇和水的溶剂化结构和分子相互作用:理论研究
摘要 采用分子动力学 (MD) 模拟系统研究了布洛芬与乙醇或水在 298.15 K 至 333.15 K 温度范围内的相互作用和溶剂化结构。结果表明布洛芬分子在乙醇中主要作为 HB 供体,而在水中通过在连续的封闭循环中与水分子建立两个 HBs,作为 HB 供体和 HB 受体。很明显,氢键 (HBs) 的强度按以下顺序逐渐减弱:布洛芬 - 乙醇 > 乙醇 - 乙醇 > 布洛芬 - 布洛芬(在布洛芬 - 乙醇混合物中),布洛芬 - 布洛芬 > 布洛芬 - 水 > 水 - 水(在布洛芬 - 乙醇混合物中)布洛芬-水混合物)。HBs随着温度升高而减弱,而HB结构几乎保持不变。水分子中大量的HBs阻碍了布洛芬的引入。此外,静电和范德华相互作用,以及布洛芬与乙醇和水的溶剂化结构几乎不受温度影响。
更新日期:2020-04-01
中文翻译:

布洛芬与乙醇和水的溶剂化结构和分子相互作用:理论研究
摘要 采用分子动力学 (MD) 模拟系统研究了布洛芬与乙醇或水在 298.15 K 至 333.15 K 温度范围内的相互作用和溶剂化结构。结果表明布洛芬分子在乙醇中主要作为 HB 供体,而在水中通过在连续的封闭循环中与水分子建立两个 HBs,作为 HB 供体和 HB 受体。很明显,氢键 (HBs) 的强度按以下顺序逐渐减弱:布洛芬 - 乙醇 > 乙醇 - 乙醇 > 布洛芬 - 布洛芬(在布洛芬 - 乙醇混合物中),布洛芬 - 布洛芬 > 布洛芬 - 水 > 水 - 水(在布洛芬 - 乙醇混合物中)布洛芬-水混合物)。HBs随着温度升高而减弱,而HB结构几乎保持不变。水分子中大量的HBs阻碍了布洛芬的引入。此外,静电和范德华相互作用,以及布洛芬与乙醇和水的溶剂化结构几乎不受温度影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号