当前位置:
X-MOL 学术
›
J. Photochem. Photobiol. B Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photolysis of thiochrome in aqueous solution: A kinetic study.
Journal of Photochemistry and Photobiology B: Biology ( IF 3.9 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.jphotobiol.2019.111766 Zubair Anwar 1 , Muhammad Ali Sheraz 1 , Sofia Ahmed 1 , Nafeesa Mustaan 1 , Adeela Khurshid 1 , Wajiha Gul 2 , Saif-Ur-Rehman Khattak 3 , Iqbal Ahmad 1
Journal of Photochemistry and Photobiology B: Biology ( IF 3.9 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.jphotobiol.2019.111766 Zubair Anwar 1 , Muhammad Ali Sheraz 1 , Sofia Ahmed 1 , Nafeesa Mustaan 1 , Adeela Khurshid 1 , Wajiha Gul 2 , Saif-Ur-Rehman Khattak 3 , Iqbal Ahmad 1
Affiliation
|
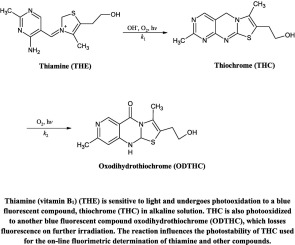
The photolysis of thiochrome (THC), an oxidation product of thiamine (vitamin B1) (THE), used for its fluorimetric assay, has been studied in the pH range 7.0-12.0. THC undergoes photooxidation to oxodihydrothiochrome (ODTHC) which is oxidized to a non-fluorescent compound (OP1) on UV irradiation. The kinetics of the consecutive first-order reactions: THC→k1ODTHC→k2OP1, has been evaluated and the values of first-order rate constants, k1 (0.58-4.20 × 10-5, s-1) and k2 (0.05-2.03 × 10-5, s-1), at pH 7.0-12.0 have been determined. The rates of degradation of THC and ODTHC are enhanced with pH and the second-order rate constants k1' and k2' for the OH- ion-catalyzed reaction are in the range of 0.002-58.3 M-1 s-1. The quantum yields of the photolysis of THC and ODTHC in the pH range 7.0-12.0 have been determined. THC, ODTHC and OP1 have been identified by chromatographic, spectrometric and fluorimetric methods. THC and ODTHC have similar fluorescence characteristics and emit at 450 and 445 nm, respectively. THC, ODTHC and OP1 with distinct absorption maxima (370, 344 and 290 nm, respectively) have been determined by a newly developed and validated multicomponent spectrometric method during the photolysis reactions. The on-line formation of THC by the photooxidation of THE may lead to the degradation of THC and give erroneous results in the fluorimetric assay of THE. A scheme for the photolysis reactions of THC in aqueous solution is presented.
中文翻译:

水溶液中硫色素的光解:动力学研究。
已在pH值7.0-12.0范围内研究了硫胺素(维生素B1)(THE)的氧化产物硫代色素(THC)的光解。THC经历光氧化为氧二氢硫代色素(ODTHC),然后在紫外线照射下被氧化为非荧光化合物(OP1)。已评估了连续一级反应的动力学:THC→k1ODTHC→k2OP1,一级速率常数k1(0.58-4.20×10-5,s-1)和k2(0.05-2.03×已确定在pH 7.0-12.0下的10-5,s-1)。pH值提高了THC和ODTHC的降解速率,OH离子催化反应的二级速率常数k1'和k2'在0.002-58.3 M-1 s-1的范围内。已确定在7.0-12.0的pH范围内THC和ODTHC光解的量子产率。THC,ODTHC和OP1已通过色谱,光谱和荧光法鉴定。THC和ODTHC具有相似的荧光特性,分别在450和445 nm处发射。在光解反应过程中,通过新开发和验证的多组分光谱法测定了具有明显不同吸收最大值(分别为370、344和290 nm)的THC,ODTCC和OP1。TH的光氧化在线形成THC可能导致THC降解,并在THE的荧光测定中给出错误的结果。提出了THC在水溶液中的光解反应方案。在光解反应过程中,已通过新开发并验证的多组分光谱法测定了具有最大吸收最大值(分别为370、344和290 nm)的ODTHC和OP1。TH的光氧化在线形成THC可能导致THC降解,并在THE的荧光测定中给出错误的结果。提出了THC在水溶液中的光解反应方案。在光解反应过程中,已通过新开发并验证的多组分光谱法测定了具有最大吸收最大值(分别为370、344和290 nm)的ODTHC和OP1。TH的光氧化在线形成THC可能导致THC降解,并在THE的荧光测定中给出错误的结果。提出了THC在水溶液中的光解反应方案。
更新日期:2020-01-04
中文翻译:

水溶液中硫色素的光解:动力学研究。
已在pH值7.0-12.0范围内研究了硫胺素(维生素B1)(THE)的氧化产物硫代色素(THC)的光解。THC经历光氧化为氧二氢硫代色素(ODTHC),然后在紫外线照射下被氧化为非荧光化合物(OP1)。已评估了连续一级反应的动力学:THC→k1ODTHC→k2OP1,一级速率常数k1(0.58-4.20×10-5,s-1)和k2(0.05-2.03×已确定在pH 7.0-12.0下的10-5,s-1)。pH值提高了THC和ODTHC的降解速率,OH离子催化反应的二级速率常数k1'和k2'在0.002-58.3 M-1 s-1的范围内。已确定在7.0-12.0的pH范围内THC和ODTHC光解的量子产率。THC,ODTHC和OP1已通过色谱,光谱和荧光法鉴定。THC和ODTHC具有相似的荧光特性,分别在450和445 nm处发射。在光解反应过程中,通过新开发和验证的多组分光谱法测定了具有明显不同吸收最大值(分别为370、344和290 nm)的THC,ODTCC和OP1。TH的光氧化在线形成THC可能导致THC降解,并在THE的荧光测定中给出错误的结果。提出了THC在水溶液中的光解反应方案。在光解反应过程中,已通过新开发并验证的多组分光谱法测定了具有最大吸收最大值(分别为370、344和290 nm)的ODTHC和OP1。TH的光氧化在线形成THC可能导致THC降解,并在THE的荧光测定中给出错误的结果。提出了THC在水溶液中的光解反应方案。在光解反应过程中,已通过新开发并验证的多组分光谱法测定了具有最大吸收最大值(分别为370、344和290 nm)的ODTHC和OP1。TH的光氧化在线形成THC可能导致THC降解,并在THE的荧光测定中给出错误的结果。提出了THC在水溶液中的光解反应方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号