当前位置:
X-MOL 学术
›
Nitric Oxide
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cellular microdomains for nitric oxide signaling in endothelium and red blood cells.
Nitric Oxide ( IF 3.2 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.niox.2020.01.002 Francesca Leo 1 , Beate Hutzler 1 , Claire A Ruddiman 2 , Brant E Isakson 3 , Miriam M Cortese-Krott 1
Nitric Oxide ( IF 3.2 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.niox.2020.01.002 Francesca Leo 1 , Beate Hutzler 1 , Claire A Ruddiman 2 , Brant E Isakson 3 , Miriam M Cortese-Krott 1
Affiliation
|
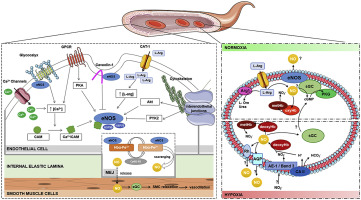
There is accumulating evidence that biological membranes are not just homogenous lipid structures, but are highly organized in microdomains, i.e. compartmentalized areas of protein and lipid complexes, which facilitate necessary interactions for various signaling pathways. Each microdomain exhibits unique composition, membrane location and dynamics, which ultimately shape their functional characteristics. In the vasculature, microdomains are crucial for organizing and compartmentalizing vasodilatory signals that contribute to blood pressure homeostasis. In this review we aim to describe how membrane microdomains in both the endothelium and red blood cells allow context-specific regulation of the vasodilatory signal nitric oxide (NO) and its corresponding metabolic products, and how this results in tightly controlled systemic physiological responses. We will describe (1) structural characteristics of microdomains including lipid rafts and caveolae; (2) endothelial cell caveolae and how they participate in mechanosensing and NO-dependent mechanotransduction; (3) the myoendothelial junction of resistance arterial endothelial cells and how protein-protein interactions within it have profound systemic effects on blood pressure regulation, and (4) putative/proposed NO microdomains in RBCs and how they participate in control of systemic NO bioavailability. The sum of these discussions will provide a current view of NO regulation by cellular microdomains.
中文翻译:

一氧化氮在内皮细胞和红细胞中的细胞微区。
越来越多的证据表明,生物膜不仅是同质的脂质结构,而且在微区(即蛋白质和脂质复合物的分隔区域)中高度组织化,从而促进了各种信号通路的必要相互作用。每个微区都具有独特的成分,膜位置和动力学,最终决定了它们的功能特性。在脉管系统中,微区对于组织和分隔有助于血压稳态的血管舒张信号至关重要。在这篇综述中,我们旨在描述内皮细胞和红细胞中的膜微结构域如何允许血管舒张信号一氧化氮(NO)及其相应代谢产物的背景特异性调节,以及如何导致严格控制的全身生理反应。我们将描述(1)包括脂质筏和小窝的微区的结构特征;(2)内皮细胞小窝以及它们如何参与机械传感和NO依赖性机械转导;(3)抵抗性动脉内皮细胞的心肌内皮连接及其中的蛋白质-蛋白质相互作用如何对血压调节产生深远的系统性影响,以及(4)RBC中假定的/提议的NO微结构域以及它们如何参与系统性NO生物利用度的控制。这些讨论的总和将提供细胞微域对NO调节的当前观点。(2)内皮细胞小窝以及它们如何参与机械传感和依赖于NO的机械转导;(3)抵抗性动脉内皮细胞的心肌内皮连接及其中的蛋白质-蛋白质相互作用如何对血压调节产生深远的系统性影响,以及(4)RBC中假定的/提议的NO微结构域以及它们如何参与系统性NO生物利用度的控制。这些讨论的总和将提供细胞微域对NO调节的当前观点。(2)内皮细胞小窝以及它们如何参与机械传感和依赖于NO的机械转导;(3)抵抗性动脉内皮细胞的心肌内皮连接及其中的蛋白质-蛋白质相互作用如何对血压调节产生深远的系统性影响,以及(4)RBC中假定的/提议的NO微结构域以及它们如何参与系统性NO生物利用度的控制。这些讨论的总和将提供细胞微域对NO调节的当前观点。
更新日期:2020-01-04
中文翻译:

一氧化氮在内皮细胞和红细胞中的细胞微区。
越来越多的证据表明,生物膜不仅是同质的脂质结构,而且在微区(即蛋白质和脂质复合物的分隔区域)中高度组织化,从而促进了各种信号通路的必要相互作用。每个微区都具有独特的成分,膜位置和动力学,最终决定了它们的功能特性。在脉管系统中,微区对于组织和分隔有助于血压稳态的血管舒张信号至关重要。在这篇综述中,我们旨在描述内皮细胞和红细胞中的膜微结构域如何允许血管舒张信号一氧化氮(NO)及其相应代谢产物的背景特异性调节,以及如何导致严格控制的全身生理反应。我们将描述(1)包括脂质筏和小窝的微区的结构特征;(2)内皮细胞小窝以及它们如何参与机械传感和NO依赖性机械转导;(3)抵抗性动脉内皮细胞的心肌内皮连接及其中的蛋白质-蛋白质相互作用如何对血压调节产生深远的系统性影响,以及(4)RBC中假定的/提议的NO微结构域以及它们如何参与系统性NO生物利用度的控制。这些讨论的总和将提供细胞微域对NO调节的当前观点。(2)内皮细胞小窝以及它们如何参与机械传感和依赖于NO的机械转导;(3)抵抗性动脉内皮细胞的心肌内皮连接及其中的蛋白质-蛋白质相互作用如何对血压调节产生深远的系统性影响,以及(4)RBC中假定的/提议的NO微结构域以及它们如何参与系统性NO生物利用度的控制。这些讨论的总和将提供细胞微域对NO调节的当前观点。(2)内皮细胞小窝以及它们如何参与机械传感和依赖于NO的机械转导;(3)抵抗性动脉内皮细胞的心肌内皮连接及其中的蛋白质-蛋白质相互作用如何对血压调节产生深远的系统性影响,以及(4)RBC中假定的/提议的NO微结构域以及它们如何参与系统性NO生物利用度的控制。这些讨论的总和将提供细胞微域对NO调节的当前观点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号