当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of magnetic MgO/Fe3O4 via the green method for competitive removal of Pb and Cd from aqueous solution
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.colsurfa.2020.124419 Mahdieh Namvar-Mahboub , Elahe Khodeir , Moein Bahadori , Seyed Mostafa Mahdizadeh
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.colsurfa.2020.124419 Mahdieh Namvar-Mahboub , Elahe Khodeir , Moein Bahadori , Seyed Mostafa Mahdizadeh
|
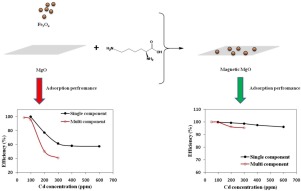
Abstract The current work focused on the development of magnesium oxide – based adsorbent for heavy metal removal. Magnetic MgO/Fe3O4 composite was prepared using lysine as a binding agent. The as-prepared adsorbents were characterized using Fourier transform infrared (FTIR) spectroscopy, Transmission electron microscopy (TEM), zeta potential, x-ray diffraction (XRD), Vibrating sample magnetometer (VSM), and specific surface measurement. The adsorption efficiency of MgO, Lysine-modified MgO and magnetic MgO /Fe3O4 composite were determined through Pb(II) and Cd(II) removal from aqueous solution. The effect of pH (2–12), adsorbent dosage (0.3–1.5 g/l) and contact time on adsorption efficiency were investigated. The results showed that maximum efficiency was obtained in the presence of 0.5 g/l of the adsorbent. Additionally, it was found that the pseudo second-order model interpreted the kinetic of adsorption on as-prepared adsorbents. Competitive adsorption of Pb(II)-Cd(II) multi-component solution were studied at different feed concentration. Compared to single-component systems, the adsorption efficiency of Cd(II) was reduced by 33, 19, and 4 % for MgO, lysine modified MgO and magnetic MgO/Fe3O4 composite, respectively. For adsorption of Pb(II) ion, Cd(II) showed inhibition effect only on lysine-modified MgO. The experimental competitive adsorption data were adequately fitted on extended Langmuir isotherm.
中文翻译:

绿色法制备磁性 MgO/Fe3O4 竞争去除水溶液中的 Pb 和 Cd
摘要 目前的工作重点是开发用于去除重金属的氧化镁基吸附剂。使用赖氨酸作为粘合剂制备磁性 MgO/Fe3O4 复合材料。使用傅里叶变换红外 (FTIR) 光谱、透射电子显微镜 (TEM)、zeta 电位、X 射线衍射 (XRD)、振动样品磁强计 (VSM) 和比表面测量对制备的吸附剂进行表征。通过从水溶液中去除 Pb(II) 和 Cd(II) 来确定 MgO、赖氨酸修饰的 MgO 和磁性 MgO/Fe3O4 复合材料的吸附效率。研究了 pH (2-12)、吸附剂剂量 (0.3-1.5 g/l) 和接触时间对吸附效率的影响。结果表明,在存在 0.5 g/l 吸附剂时获得最大效率。此外,发现伪二级模型解释了在制备的吸附剂上的吸附动力学。研究了不同进料浓度下 Pb(II)-Cd(II) 多组分溶液的竞争吸附。与单组分系统相比,MgO、赖氨酸改性的 MgO 和磁性 MgO/Fe3O4 复合物对 Cd(II) 的吸附效率分别降低了 33%、19% 和 4%。对于 Pb(II) 离子的吸附,Cd(II) 仅对赖氨酸修饰的 MgO 显示出抑制作用。实验竞争吸附数据充分拟合扩展朗缪尔等温线。对于 MgO、赖氨酸改性的 MgO 和磁性 MgO/Fe3O4 复合材料,Cd(II) 的吸附效率分别降低了 33%、19% 和 4%。对于 Pb(II) 离子的吸附,Cd(II) 仅对赖氨酸修饰的 MgO 显示出抑制作用。实验竞争吸附数据充分拟合扩展朗缪尔等温线。对于 MgO、赖氨酸改性的 MgO 和磁性 MgO/Fe3O4 复合材料,Cd(II) 的吸附效率分别降低了 33%、19% 和 4%。对于 Pb(II) 离子的吸附,Cd(II) 仅对赖氨酸修饰的 MgO 显示出抑制作用。实验竞争吸附数据充分拟合扩展朗缪尔等温线。
更新日期:2020-02-01
中文翻译:

绿色法制备磁性 MgO/Fe3O4 竞争去除水溶液中的 Pb 和 Cd
摘要 目前的工作重点是开发用于去除重金属的氧化镁基吸附剂。使用赖氨酸作为粘合剂制备磁性 MgO/Fe3O4 复合材料。使用傅里叶变换红外 (FTIR) 光谱、透射电子显微镜 (TEM)、zeta 电位、X 射线衍射 (XRD)、振动样品磁强计 (VSM) 和比表面测量对制备的吸附剂进行表征。通过从水溶液中去除 Pb(II) 和 Cd(II) 来确定 MgO、赖氨酸修饰的 MgO 和磁性 MgO/Fe3O4 复合材料的吸附效率。研究了 pH (2-12)、吸附剂剂量 (0.3-1.5 g/l) 和接触时间对吸附效率的影响。结果表明,在存在 0.5 g/l 吸附剂时获得最大效率。此外,发现伪二级模型解释了在制备的吸附剂上的吸附动力学。研究了不同进料浓度下 Pb(II)-Cd(II) 多组分溶液的竞争吸附。与单组分系统相比,MgO、赖氨酸改性的 MgO 和磁性 MgO/Fe3O4 复合物对 Cd(II) 的吸附效率分别降低了 33%、19% 和 4%。对于 Pb(II) 离子的吸附,Cd(II) 仅对赖氨酸修饰的 MgO 显示出抑制作用。实验竞争吸附数据充分拟合扩展朗缪尔等温线。对于 MgO、赖氨酸改性的 MgO 和磁性 MgO/Fe3O4 复合材料,Cd(II) 的吸附效率分别降低了 33%、19% 和 4%。对于 Pb(II) 离子的吸附,Cd(II) 仅对赖氨酸修饰的 MgO 显示出抑制作用。实验竞争吸附数据充分拟合扩展朗缪尔等温线。对于 MgO、赖氨酸改性的 MgO 和磁性 MgO/Fe3O4 复合材料,Cd(II) 的吸附效率分别降低了 33%、19% 和 4%。对于 Pb(II) 离子的吸附,Cd(II) 仅对赖氨酸修饰的 MgO 显示出抑制作用。实验竞争吸附数据充分拟合扩展朗缪尔等温线。











































 京公网安备 11010802027423号
京公网安备 11010802027423号