当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Myeloid Cell-Targeted Nanocarriers Efficiently Inhibit Cellular Inhibitor of Apoptosis for Cancer Immunotherapy.
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-01-02 , DOI: 10.1016/j.chembiol.2019.12.007 Peter D Koch 1 , Christopher B Rodell 1 , Rainer H Kohler 1 , Mikael J Pittet 1 , Ralph Weissleder 2
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-01-02 , DOI: 10.1016/j.chembiol.2019.12.007 Peter D Koch 1 , Christopher B Rodell 1 , Rainer H Kohler 1 , Mikael J Pittet 1 , Ralph Weissleder 2
Affiliation
|
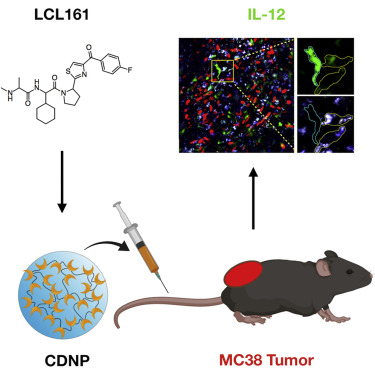
Immune-checkpoint blockers can promote sustained clinical responses in a subset of cancer patients. Recent research has shown that a subpopulation of tumor-infiltrating dendritic cells functions as gatekeepers, sensitizing tumors to anti-PD-1 treatment via production of interleukin-12 (IL-12). Hypothesizing that myeloid cell-targeted nanomaterials could be used to deliver small-molecule IL-12 inducers, we performed high-content image-based screening to identify the most efficacious small-molecule compounds. Using one lead candidate, LCL161, we created a myeloid-targeted nanoformulation that induced IL-12 production in intratumoral myeloid cells in vivo, slowed tumor growth as a monotherapy, and had no significant systemic toxicity. These results pave the way for developing combination immunotherapeutics by harnessing IL-12 production for immunostimulation.
中文翻译:

靶向髓样细胞的纳米载体可有效抑制细胞凋亡的细胞抑制剂,用于癌症免疫治疗。
免疫检查点阻滞剂可以促进一部分癌症患者的持续临床反应。最近的研究表明,肿瘤浸润树突状细胞亚群起着看门人的作用,通过产生白介素12(IL-12)使肿瘤对抗PD-1治疗敏感。推测髓样细胞靶向的纳米材料可用于递送小分子IL-12诱导剂,我们进行了基于图像的高含量筛选,以鉴定最有效的小分子化合物。使用一种潜在的候选药物LCL161,我们创建了一种靶向髓样的纳米制剂,该纳米制剂可在体内诱导肿瘤内髓样细胞中IL-12的产生,作为一种单一疗法可减缓肿瘤的生长,并且没有明显的全身毒性。
更新日期:2020-01-04
中文翻译:

靶向髓样细胞的纳米载体可有效抑制细胞凋亡的细胞抑制剂,用于癌症免疫治疗。
免疫检查点阻滞剂可以促进一部分癌症患者的持续临床反应。最近的研究表明,肿瘤浸润树突状细胞亚群起着看门人的作用,通过产生白介素12(IL-12)使肿瘤对抗PD-1治疗敏感。推测髓样细胞靶向的纳米材料可用于递送小分子IL-12诱导剂,我们进行了基于图像的高含量筛选,以鉴定最有效的小分子化合物。使用一种潜在的候选药物LCL161,我们创建了一种靶向髓样的纳米制剂,该纳米制剂可在体内诱导肿瘤内髓样细胞中IL-12的产生,作为一种单一疗法可减缓肿瘤的生长,并且没有明显的全身毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号