当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C6-O-alkylated 7-deazainosine nucleoside analogues: Discovery of potent and selective anti-sleeping sickness agents.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.ejmech.2019.112018 Fabian Hulpia 1 , Jakob Bouton 1 , Gustavo D Campagnaro 2 , Ibrahim A Alfayez 2 , Dorien Mabille 3 , Louis Maes 3 , Harry P de Koning 2 , Guy Caljon 3 , Serge Van Calenbergh 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.ejmech.2019.112018 Fabian Hulpia 1 , Jakob Bouton 1 , Gustavo D Campagnaro 2 , Ibrahim A Alfayez 2 , Dorien Mabille 3 , Louis Maes 3 , Harry P de Koning 2 , Guy Caljon 3 , Serge Van Calenbergh 1
Affiliation
|
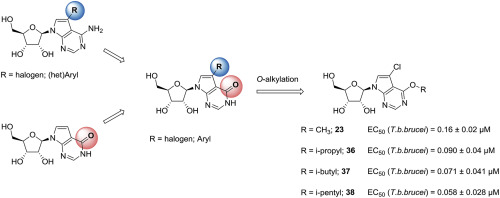
African trypanosomiasis, a deadly infectious disease caused by the protozoan Trypanosoma brucei spp., is spread to new hosts by bites of infected tsetse flies. Currently approved therapies all have their specific drawbacks, prompting a search for novel therapeutic agents. T. brucei lacks the enzymes necessary to forge the purine ring from amino acid precursors, rendering them dependent on the uptake and interconversion of host purines. This dependency renders analogues of purines and corresponding nucleosides an interesting source of potential anti-T. brucei agents. In this study, we synthesized and evaluated a series of 7-substituted 7-deazainosine derivatives and found that 6-O-alkylated analogues in particular showed highly promising in vitro activity with EC50 values in the mid-nanomolar range. SAR investigation of the O-alkyl chain showed that antitrypanosomal activity increased, and also cytotoxicity, with alkyl chain length, at least in the linear alkyl chain series. However, this could be attenuated by introducing a terminal branch point, resulting in the highly potent and selective analogues, 36, 37 and 38. No resistance related to transporter-mediated uptake could be identified, earmarking several of these analogues for further in vivo follow-up studies.
中文翻译:

C6-O-烷基化的7-脱氮芥子苷核苷类似物:发现有效的和选择性的抗睡眠病药物。
非洲锥虫病是由原生动物Trypanosoma brucei spp。引起的一种致命的传染病,通过被感染的采采蝇的叮咬传播到新的寄主。目前批准的疗法都有其特定的缺点,促使人们寻找新的治疗剂。T. brucei缺乏从氨基酸前体形成嘌呤环所需的酶,使其依赖于宿主嘌呤的吸收和相互转化。这种依赖性使得嘌呤的类似物和相应的核苷成为潜在的抗-T的有趣来源。布鲁斯代理商。在这项研究中,我们合成和评估了一系列7-取代的7-脱氮芥子碱衍生物,发现6-O-烷基化的类似物特别具有极好的前景,其EC50值在纳摩尔级范围内。SAR对O-烷基链的研究表明,至少在线性烷基链系列中,随着烷基链长度的增加,抗锥虫活性以及细胞毒性也随之增加。然而,这可以通过引入末端分支点来减弱,从而产生高度有效和选择性的类似物36、37和38。无法鉴定出与转运蛋白介导的摄取相关的抗药性,这些类似物中的几种被指定用于进一步的体内跟踪研究。研究。
更新日期:2020-01-04
中文翻译:

C6-O-烷基化的7-脱氮芥子苷核苷类似物:发现有效的和选择性的抗睡眠病药物。
非洲锥虫病是由原生动物Trypanosoma brucei spp。引起的一种致命的传染病,通过被感染的采采蝇的叮咬传播到新的寄主。目前批准的疗法都有其特定的缺点,促使人们寻找新的治疗剂。T. brucei缺乏从氨基酸前体形成嘌呤环所需的酶,使其依赖于宿主嘌呤的吸收和相互转化。这种依赖性使得嘌呤的类似物和相应的核苷成为潜在的抗-T的有趣来源。布鲁斯代理商。在这项研究中,我们合成和评估了一系列7-取代的7-脱氮芥子碱衍生物,发现6-O-烷基化的类似物特别具有极好的前景,其EC50值在纳摩尔级范围内。SAR对O-烷基链的研究表明,至少在线性烷基链系列中,随着烷基链长度的增加,抗锥虫活性以及细胞毒性也随之增加。然而,这可以通过引入末端分支点来减弱,从而产生高度有效和选择性的类似物36、37和38。无法鉴定出与转运蛋白介导的摄取相关的抗药性,这些类似物中的几种被指定用于进一步的体内跟踪研究。研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号