Tetrahedron ( IF 2.1 ) Pub Date : 2020-01-03 , DOI: 10.1016/j.tet.2019.130918 Arindam Khatua , Avishek Roy , Vishnumaya Bisai
|
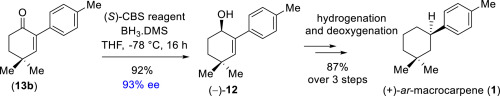
Concise catalytic asymmetric total syntheses of aromatic sesquiterpenes, naturally occurring (+)-ar-macrocarpene (1) and unnatural (−)-ar-macrocarpene (ent-1) have been featured (6 steps, ∼60–61% overall yields) from commercially available 4,4-dimethylcyclohex-2-enone 16. The enantioenriched allyl alcohol 12 is obtained from catalytic enantioselective reduction of enone 13b using Corey-Bakshi-Shibata catalyst in 93% ee, which is found to be the key intermediate. A highly diastereoselective hydrogenation of enantioenriched allyl alcohol 12 (10.3:1 dr) followed by deoxygenation completes straightforward access to (+)-ar-macrocarpene (1) and (−)-ar-macrocarpene (ent-1).
中文翻译:

倍半萜,(+)-和(-)- ar-大麦烯的催化不对称全合成
精巧地合成了芳香倍半萜的催化不对称全合成,包括天然(+)- ar-大麦烯(1)和非天然(-)- ar-大麦烯(ent - 1)(6步,总产率约60-61%)从市售的4,4-二甲基环己-2-烯酮16。通过使用Corey-Bakshi-Shibata催化剂在93%ee中催化烯酮13b的催化对映选择性还原获得对映体富集的烯丙醇12,发现其为关键中间体。对映体富集的烯丙醇的高度非对映选择性氢化12(10.3:1 dr),然后进行脱氧操作,可以轻松地直接访问(+)- ar-大麦烯(1)和(-)- ar-大麦烯(ent - 1)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号