当前位置:
X-MOL 学术
›
PLOS Pathog.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
USP12 translocation maintains interferon antiviral efficacy by inhibiting CBP acetyltransferase activity.
PLoS Pathogens ( IF 5.5 ) Pub Date : 2020-01-03 , DOI: 10.1371/journal.ppat.1008215 Jin Liu 1, 2, 3 , Lincong Jin 1, 2 , Xiangjie Chen 1, 2 , Yukang Yuan 1, 2 , Yibo Zuo 1, 2 , Ying Miao 1, 2 , Qian Feng 1, 2 , Hongguang Zhang 1, 2 , Fan Huang 1, 2 , Tingting Guo 1, 2 , Liting Zhang 1, 2 , Li Zhu 3 , Feng Qian 3 , Chuanwu Zhu 3 , Hui Zheng 1, 2
PLoS Pathogens ( IF 5.5 ) Pub Date : 2020-01-03 , DOI: 10.1371/journal.ppat.1008215 Jin Liu 1, 2, 3 , Lincong Jin 1, 2 , Xiangjie Chen 1, 2 , Yukang Yuan 1, 2 , Yibo Zuo 1, 2 , Ying Miao 1, 2 , Qian Feng 1, 2 , Hongguang Zhang 1, 2 , Fan Huang 1, 2 , Tingting Guo 1, 2 , Liting Zhang 1, 2 , Li Zhu 3 , Feng Qian 3 , Chuanwu Zhu 3 , Hui Zheng 1, 2
Affiliation
|
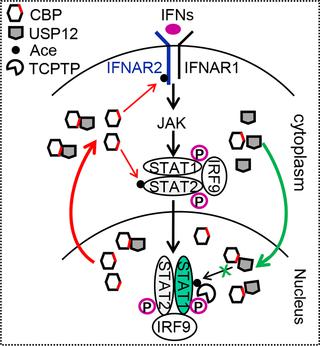
CREB-binding protein (CBP) participates in numerous transcription events. However, cell-intrinsic inhibitors of CBP are poorly defined. Here, we found that cellular USP12 interacts with the HAT domain of CBP and inhibits CBP's acetyltransferase activity. Interestingly, USP12 positively regulates interferon (IFN) antiviral signaling independently of its deubiquitinase activity. Furthermore, we found that in IFN signaling USP12 translocates from the cytoplasm to the nucleus. The decrease in cytoplasmic USP12 facilitates CBP-induced acetylation and activation of IFN signaling proteins in the cytoplasm. Moreover, USP12 accumulation in the nucleus blocks CBP-induced acetylation of phosphorylated STAT1 (p-STAT1) and therefore inhibits the dephosphorylation effects of TCPTP on p-STAT1, which finally maintains nuclear p-STAT1 levels and IFN antiviral efficacy. USP12 nuclear translocation extends our understanding of the regulation of the strength of IFN antiviral signaling. Our study uncovers a cell-intrinsic regulation of CBP acetyltransferase activity and may provide potential strategies for IFN-based antiviral therapy.
中文翻译:

USP12易位通过抑制CBP乙酰转移酶活性来维持干扰素抗病毒功效。
CREB结合蛋白(CBP)参与许多转录事件。但是,细胞内CBP抑制剂的定义不明确。在这里,我们发现细胞USP12与CBP的HAT结构域相互作用,并抑制CBP的乙酰转移酶活性。有趣的是,USP12独立于其去泛素酶活性而积极调节干扰素(IFN)抗病毒信号。此外,我们发现在IFN信号传导中,USP12从细胞质转移到细胞核。细胞质USP12的减少促进了CBP诱导的乙酰化和细胞质中IFN信号蛋白的激活。此外,USP12在核中的蓄积会阻止CBP诱导的磷酸化STAT1(p-STAT1)乙酰化,因此抑制了TCPTP对p-STAT1的去磷酸化作用,最终维持核p-STAT1水平和IFN抗病毒功效。USP12核易位扩展了我们对IFN抗病毒信号强度调节的理解。我们的研究揭示了CBP乙酰转移酶活性的细胞内在调节,并可能为基于IFN的抗病毒治疗提供潜在策略。
更新日期:2020-01-04
中文翻译:

USP12易位通过抑制CBP乙酰转移酶活性来维持干扰素抗病毒功效。
CREB结合蛋白(CBP)参与许多转录事件。但是,细胞内CBP抑制剂的定义不明确。在这里,我们发现细胞USP12与CBP的HAT结构域相互作用,并抑制CBP的乙酰转移酶活性。有趣的是,USP12独立于其去泛素酶活性而积极调节干扰素(IFN)抗病毒信号。此外,我们发现在IFN信号传导中,USP12从细胞质转移到细胞核。细胞质USP12的减少促进了CBP诱导的乙酰化和细胞质中IFN信号蛋白的激活。此外,USP12在核中的蓄积会阻止CBP诱导的磷酸化STAT1(p-STAT1)乙酰化,因此抑制了TCPTP对p-STAT1的去磷酸化作用,最终维持核p-STAT1水平和IFN抗病毒功效。USP12核易位扩展了我们对IFN抗病毒信号强度调节的理解。我们的研究揭示了CBP乙酰转移酶活性的细胞内在调节,并可能为基于IFN的抗病毒治疗提供潜在策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号