Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Streptavidin Homologues for Applications on Solid Surfaces at High Temperatures.
Langmuir ( IF 3.9 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.langmuir.9b02339 Carsten Schmidt 1 , Peter Schierack 1 , Ulrike Gerber 1 , Christian Schröder 1 , Youngeun Choi 2, 3 , Ilko Bald 2, 3 , Werner Lehmann 4 , Stefan Rödiger 1, 5
Langmuir ( IF 3.9 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.langmuir.9b02339 Carsten Schmidt 1 , Peter Schierack 1 , Ulrike Gerber 1 , Christian Schröder 1 , Youngeun Choi 2, 3 , Ilko Bald 2, 3 , Werner Lehmann 4 , Stefan Rödiger 1, 5
Affiliation
|
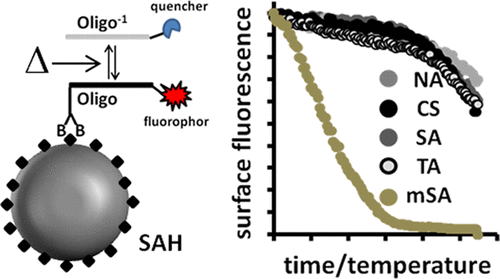
One of the most commonly used bonds between two biomolecules is the bond between biotin and streptavidin (SA) or streptavidin homologues (SAHs). A high dissociation constant and the consequent high-temperature stability even allows for its use in nucleic acid detection under polymerase chain reaction (PCR) conditions. There are a number of SAHs available, and for assay design, it is of great interest to determine as to which SAH will perform the best under assay conditions. Although there are numerous single studies on the characterization of SAHs in solution or selected solid phases, there is no systematic study comparing different SAHs for biomolecule-binding, hybridization, and PCR assays on solid phases. We compared streptavidin, core streptavidin, traptavidin, core traptavidin, neutravidin, and monomeric streptavidin on the surface of microbeads (10-15 μm in diameter) and designed multiplex microbead-based experiments and analyzed simultaneously the binding of biotinylated oligonucleotides and the hybridization of oligonucleotides to complementary capture probes. We also bound comparably large DNA origamis to capture probes on the microbead surface. We used a real-time fluorescence microscopy imaging platform, with which it is possible to subject samples to a programmable time and temperature profile and to record binding processes on the microbead surface depending on the time and temperature. With the exception of core traptavidin and monomeric streptavidin, all other SA/SAHs were suitable for our investigations. We found hybridization efficiencies close to 100% for streptavidin, core streptavidin, traptavidin, and neutravidin. These could all be considered equally suitable for hybridization, PCR applications, and melting point analysis. The SA/SAH-biotin bond was temperature-sensitive when the oligonucleotide was mono-biotinylated, with traptavidin being the most stable followed by streptavidin and neutravidin. Mono-biotinylated oligonucleotides can be used in experiments with temperatures up to 70 °C. When oligonucleotides were bis-biotinylated, all SA/SAH-biotin bonds had similar temperature stability under PCR conditions, even if they comprised a streptavidin variant with slower biotin dissociation and increased mechanostability.
中文翻译:

用于高温固体表面上的链霉亲和素同系物。
两个生物分子之间最常用的键之一是生物素与链霉亲和素(SA)或链霉亲和素同源物(SAH)之间的键。高解离常数和随之而来的高温稳定性甚至使其可用于聚合酶链反应(PCR)条件下的核酸检测。有许多可用的SAH,对于分析设计,确定哪种SAH在分析条件下表现最佳将引起极大的兴趣。尽管有许多关于溶液或选定固相中SAHs表征的单项研究,但尚无系统的研究将不同SAH用于固相生物分子结合,杂交和PCR分析进行比较。我们比较了链霉亲和素,核心链霉亲和素,traptavidin,核心traptavidin,neutravidin,和微珠表面上的单体链霉亲和素(直径为10-15μm),并设计了基于多重微珠的多重实验,并同时分析了生物素化寡核苷酸的结合以及寡核苷酸与互补捕获探针的杂交。我们还绑定了相当大的DNA Origamis,以捕获微珠表面上的探针。我们使用了实时荧光显微镜成像平台,利用该平台可以对样品进行可编程的时间和温度曲线分析,并根据时间和温度记录微珠表面的结合过程。除了核心特拉维维汀和单体链霉亲和素外,所有其他SA / SAHs均适合我们的研究。我们发现链霉亲和素,核心链霉亲和素,traptavidin和neutravidin的杂交效率接近100%。这些都可以认为均适用于杂交,PCR应用和熔点分析。当寡核苷酸被单生物素化时,SA / SAH-生物素键对温度敏感,其中traptavidin最稳定,其次是链霉亲和素和neutravidin。单生物素化的寡核苷酸可用于温度高达70°C的实验中。当寡核苷酸被双生物素化时,所有SA / SAH-生物素键在PCR条件下都具有相似的温度稳定性,即使它们包含链霉亲和素变体且生物素解离较慢且机械稳定性也有所提高。Tratavidin最稳定,其次是链霉亲和素和neutravidin。单生物素化的寡核苷酸可用于温度高达70°C的实验中。当寡核苷酸被双生物素化时,所有SA / SAH-生物素键在PCR条件下都具有相似的温度稳定性,即使它们包含链霉亲和素变体且生物素解离较慢且机械稳定性也有所提高。Tratavidin最稳定,其次是链霉亲和素和neutravidin。单生物素化的寡核苷酸可用于温度高达70°C的实验中。当寡核苷酸被双生物素化时,所有SA / SAH-生物素键在PCR条件下都具有相似的温度稳定性,即使它们包含链霉亲和素变体且生物素解离较慢且机械稳定性也有所提高。
更新日期:2020-01-10
中文翻译:

用于高温固体表面上的链霉亲和素同系物。
两个生物分子之间最常用的键之一是生物素与链霉亲和素(SA)或链霉亲和素同源物(SAH)之间的键。高解离常数和随之而来的高温稳定性甚至使其可用于聚合酶链反应(PCR)条件下的核酸检测。有许多可用的SAH,对于分析设计,确定哪种SAH在分析条件下表现最佳将引起极大的兴趣。尽管有许多关于溶液或选定固相中SAHs表征的单项研究,但尚无系统的研究将不同SAH用于固相生物分子结合,杂交和PCR分析进行比较。我们比较了链霉亲和素,核心链霉亲和素,traptavidin,核心traptavidin,neutravidin,和微珠表面上的单体链霉亲和素(直径为10-15μm),并设计了基于多重微珠的多重实验,并同时分析了生物素化寡核苷酸的结合以及寡核苷酸与互补捕获探针的杂交。我们还绑定了相当大的DNA Origamis,以捕获微珠表面上的探针。我们使用了实时荧光显微镜成像平台,利用该平台可以对样品进行可编程的时间和温度曲线分析,并根据时间和温度记录微珠表面的结合过程。除了核心特拉维维汀和单体链霉亲和素外,所有其他SA / SAHs均适合我们的研究。我们发现链霉亲和素,核心链霉亲和素,traptavidin和neutravidin的杂交效率接近100%。这些都可以认为均适用于杂交,PCR应用和熔点分析。当寡核苷酸被单生物素化时,SA / SAH-生物素键对温度敏感,其中traptavidin最稳定,其次是链霉亲和素和neutravidin。单生物素化的寡核苷酸可用于温度高达70°C的实验中。当寡核苷酸被双生物素化时,所有SA / SAH-生物素键在PCR条件下都具有相似的温度稳定性,即使它们包含链霉亲和素变体且生物素解离较慢且机械稳定性也有所提高。Tratavidin最稳定,其次是链霉亲和素和neutravidin。单生物素化的寡核苷酸可用于温度高达70°C的实验中。当寡核苷酸被双生物素化时,所有SA / SAH-生物素键在PCR条件下都具有相似的温度稳定性,即使它们包含链霉亲和素变体且生物素解离较慢且机械稳定性也有所提高。Tratavidin最稳定,其次是链霉亲和素和neutravidin。单生物素化的寡核苷酸可用于温度高达70°C的实验中。当寡核苷酸被双生物素化时,所有SA / SAH-生物素键在PCR条件下都具有相似的温度稳定性,即使它们包含链霉亲和素变体且生物素解离较慢且机械稳定性也有所提高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号