当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biosynthesis of Anatoxins in Cyanobacteria: Identification of the Carboxy-anatoxins as the Penultimate Biosynthetic Intermediates.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.jnatprod.9b01121 Andreja Kust 1, 2, 3 , Annick Méjean 1 , Olivier Ploux 1, 4
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.jnatprod.9b01121 Andreja Kust 1, 2, 3 , Annick Méjean 1 , Olivier Ploux 1, 4
Affiliation
|
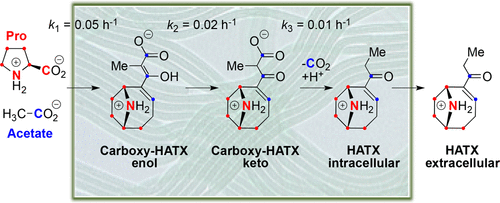
Anatoxin-a, homoanatoxin-a, and dihydroanatoxin-a are potent cyanobacterial neurotoxins. They are biosynthesized in cyanobacteria from proline and acetate by a pathway involving three polyketide synthases. We report the identification of carboxy-anatoxin-a, carboxy-homoanatoxin-a, and carboxy-dihydroanatoxin-a in acidic extracts of Cuspidothrix issatschenkoi CHARLIE-1, Oscillatoria sp. PCC 6506, and Cylindrospermum stagnale PCC 7417, respectively, using liquid chromatography coupled to mass spectrometry. The structure of these carboxy derivatives was confirmed by mass spectrometry and by isotopic incorporation experiments using labeled proline and acetate. Each of these three cyanobacteria only produce one carboxy-anatoxin, suggesting that these metabolites are the product of the hydrolysis by AnaA, the type II thioesterase, of the thioesters bound to AnaG, the last polyketide synthase of the pathway. By measuring the rate of isotopic incorporation of labeled proline into carboxy-homoanatoxin-a and homoanatoxin-a produced by Oscillatoria sp. PCC 6506, we show that carboxy-homoanatoxin-a is the intracellular precursor of homoanatoxin-a, and that homoanatoxin-a is then excreted into the extracellular medium. The transformation of carboxy-homoanatoxin-a into homoanatoxin-a is a very slow two-step process, with accumulation of carboxy-homoanatoxin-a, suggesting that the decarboxylation is spontaneous and not enzymatically catalyzed. However, an unidentified and extracellular catalyst accelerates the decarboxylation when the cell extracts are prepared at neutral pH.
中文翻译:

蓝藻中毒素的生物合成:鉴定为倒数第二个生物合成中间体的羧-毒素。
Anatoxin-a,homoanatoxin-a和dihydroanatoxin-a是有效的蓝细菌神经毒素。它们通过涉及三种聚酮化合物合酶的途径在脯氨酸和乙酸盐中蓝藻中生物合成。我们报告了在Cuspidothrix issatschenkoi CHARLIE-1,Oscillatoria sp。的酸性提取物中羧基-毒素-a,羧基-同毒素-a和羧基-二氢毒素-a的鉴定。使用液相色谱-质谱联用技术分别对PCC 6506和Stalindrospermum stagnale PCC 7417进行分析。这些羧基衍生物的结构通过质谱法和使用标记的脯氨酸和乙酸盐的同位素掺入实验来确认。这三种蓝细菌中的每一种仅产生一种羧基毒素,这表明这些代谢产物是AnaA(II型硫酯酶)水解的产物,硫酯与AnaG结合,后者是该途径的最后一个聚酮化合物合酶。通过测量同位素标记的脯氨酸掺入由Oscillatoria sp。产生的羧基同型毒素a和同型毒素a中的比率。PCC 6506,我们显示羧基同型毒素a是同型毒素a的细胞内前体,然后同型毒素a被排泄到细胞外培养基中。羧基-高毒素-a转化为高毒素-a是一个非常缓慢的两步过程,羧基-高毒素-a的积累表明脱羧是自发的而不是酶促的。但是,当在中性pH下制备细胞提取物时,身份不明的细胞外催化剂会加速脱羧反应。通过测量同位素标记的脯氨酸掺入由Oscillatoria sp。产生的羧基同型毒素a和同型毒素a中的比率。PCC 6506,我们证明了羧基-高毒素-a是高毒素-a的细胞内前体,然后高毒素-a被排泄到细胞外培养基中。羧基-高毒素-a转化为高毒素-a是一个非常缓慢的两步过程,羧基-高毒素-a的积累表明脱羧是自发的而不是酶促的。但是,当在中性pH下制备细胞提取物时,身份不明的细胞外催化剂会加速脱羧反应。通过测量同位素标记的脯氨酸掺入由Oscillatoria sp。产生的羧基同型毒素a和同型毒素a中的比率。PCC 6506,我们显示羧基同型毒素a是同型毒素a的细胞内前体,然后同型毒素a被排泄到细胞外培养基中。羧基-高毒素-a转化为高毒素-a是一个非常缓慢的两步过程,羧基-高毒素-a的积累表明脱羧是自发的而不是酶促的。但是,当在中性pH下制备细胞提取物时,身份不明的细胞外催化剂会加速脱羧反应。然后将该高毒素-a排泄到细胞外培养基中。羧基-高毒素-a转化为高毒素-a是一个非常缓慢的两步过程,羧基-高毒素-a的积累表明脱羧是自发的而不是酶促的。但是,当在中性pH下制备细胞提取物时,身份不明的细胞外催化剂会加速脱羧反应。然后将该高毒素-a排泄到细胞外培养基中。羧基-高毒素-a转化为高毒素-a是一个非常缓慢的两步过程,羧基-高毒素-a的积累表明脱羧是自发的而不是酶促的。但是,当在中性pH下制备细胞提取物时,身份不明的细胞外催化剂会加速脱羧反应。
更新日期:2020-01-04
中文翻译:

蓝藻中毒素的生物合成:鉴定为倒数第二个生物合成中间体的羧-毒素。
Anatoxin-a,homoanatoxin-a和dihydroanatoxin-a是有效的蓝细菌神经毒素。它们通过涉及三种聚酮化合物合酶的途径在脯氨酸和乙酸盐中蓝藻中生物合成。我们报告了在Cuspidothrix issatschenkoi CHARLIE-1,Oscillatoria sp。的酸性提取物中羧基-毒素-a,羧基-同毒素-a和羧基-二氢毒素-a的鉴定。使用液相色谱-质谱联用技术分别对PCC 6506和Stalindrospermum stagnale PCC 7417进行分析。这些羧基衍生物的结构通过质谱法和使用标记的脯氨酸和乙酸盐的同位素掺入实验来确认。这三种蓝细菌中的每一种仅产生一种羧基毒素,这表明这些代谢产物是AnaA(II型硫酯酶)水解的产物,硫酯与AnaG结合,后者是该途径的最后一个聚酮化合物合酶。通过测量同位素标记的脯氨酸掺入由Oscillatoria sp。产生的羧基同型毒素a和同型毒素a中的比率。PCC 6506,我们显示羧基同型毒素a是同型毒素a的细胞内前体,然后同型毒素a被排泄到细胞外培养基中。羧基-高毒素-a转化为高毒素-a是一个非常缓慢的两步过程,羧基-高毒素-a的积累表明脱羧是自发的而不是酶促的。但是,当在中性pH下制备细胞提取物时,身份不明的细胞外催化剂会加速脱羧反应。通过测量同位素标记的脯氨酸掺入由Oscillatoria sp。产生的羧基同型毒素a和同型毒素a中的比率。PCC 6506,我们证明了羧基-高毒素-a是高毒素-a的细胞内前体,然后高毒素-a被排泄到细胞外培养基中。羧基-高毒素-a转化为高毒素-a是一个非常缓慢的两步过程,羧基-高毒素-a的积累表明脱羧是自发的而不是酶促的。但是,当在中性pH下制备细胞提取物时,身份不明的细胞外催化剂会加速脱羧反应。通过测量同位素标记的脯氨酸掺入由Oscillatoria sp。产生的羧基同型毒素a和同型毒素a中的比率。PCC 6506,我们显示羧基同型毒素a是同型毒素a的细胞内前体,然后同型毒素a被排泄到细胞外培养基中。羧基-高毒素-a转化为高毒素-a是一个非常缓慢的两步过程,羧基-高毒素-a的积累表明脱羧是自发的而不是酶促的。但是,当在中性pH下制备细胞提取物时,身份不明的细胞外催化剂会加速脱羧反应。然后将该高毒素-a排泄到细胞外培养基中。羧基-高毒素-a转化为高毒素-a是一个非常缓慢的两步过程,羧基-高毒素-a的积累表明脱羧是自发的而不是酶促的。但是,当在中性pH下制备细胞提取物时,身份不明的细胞外催化剂会加速脱羧反应。然后将该高毒素-a排泄到细胞外培养基中。羧基-高毒素-a转化为高毒素-a是一个非常缓慢的两步过程,羧基-高毒素-a的积累表明脱羧是自发的而不是酶促的。但是,当在中性pH下制备细胞提取物时,身份不明的细胞外催化剂会加速脱羧反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号