当前位置:
X-MOL 学术
›
Commun. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glutathione facilitates enterovirus assembly by binding at a druggable pocket.
Communications Biology ( IF 5.2 ) Pub Date : 2020-01-03 , DOI: 10.1038/s42003-019-0722-x Helen M E Duyvesteyn 1, 2 , Jingshan Ren 1 , Thomas S Walter 1 , Elizabeth E Fry 1 , David I Stuart 1, 2
Communications Biology ( IF 5.2 ) Pub Date : 2020-01-03 , DOI: 10.1038/s42003-019-0722-x Helen M E Duyvesteyn 1, 2 , Jingshan Ren 1 , Thomas S Walter 1 , Elizabeth E Fry 1 , David I Stuart 1, 2
Affiliation
|
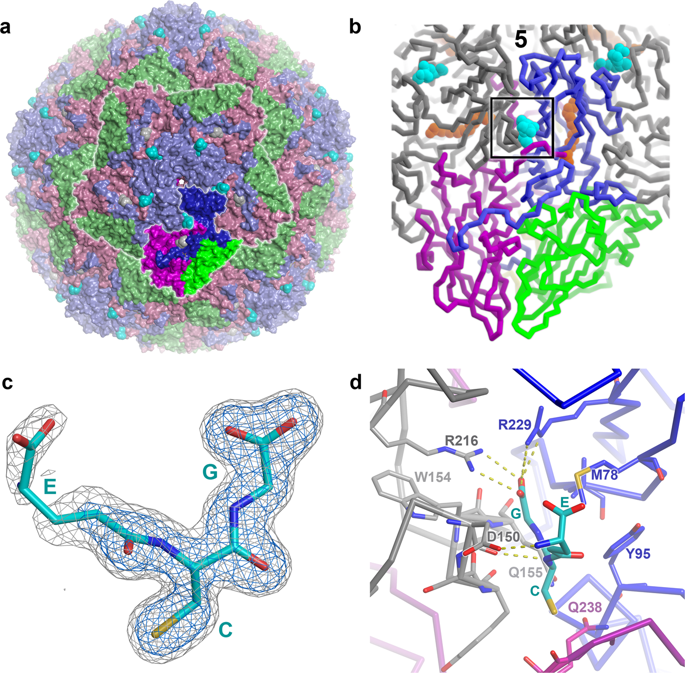
Enteroviruses cause a range of human and animal diseases, some life-threatening, but there remain no licenced anti-enterovirus drugs. However, a benzene-sulfonamide derivative and related compounds have been shown recently to block infection of a range of enteroviruses by binding the capsid at a positively-charged surface depression conserved across many enteroviruses. It has also been established that glutathione is essential for the assembly of many enteroviruses, interacting with the capsid proteins to facilitate the formation of the pentameric assembly intermediate, although the mechanism is unknown. Here we show, by high resolution structure analyses of enterovirus F3, that reduced glutathione binds to the same interprotomer pocket as the benzene-sulfonamide derivative. Bound glutathione makes strong interactions with adjacent protomers, thereby explaining the underlying biological role of this druggable binding pocket and delineating the pharmacophore for potential antivirals.
中文翻译:

谷胱甘肽通过结合在可药物口袋上促进肠道病毒组装。
肠道病毒会引起一系列人类和动物疾病,其中一些疾病危及生命,但目前还没有获得许可的抗肠道病毒药物。然而,苯磺酰胺衍生物和相关化合物最近已被证明可以通过在许多肠道病毒中保守的带正电荷的表面凹陷处结合衣壳来阻止一系列肠道病毒的感染。还确定谷胱甘肽对于许多肠道病毒的组装至关重要,它与衣壳蛋白相互作用以促进五聚体组装中间体的形成,尽管其机制尚不清楚。在这里,我们通过肠道病毒 F3 的高分辨率结构分析表明,还原型谷胱甘肽与苯磺酰胺衍生物结合到相同的原体间口袋。结合的谷胱甘肽与邻近的原聚体产生强烈的相互作用,从而解释了这种可成药结合袋的潜在生物学作用,并描绘了潜在抗病毒药物的药效团。
更新日期:2020-01-04
中文翻译:

谷胱甘肽通过结合在可药物口袋上促进肠道病毒组装。
肠道病毒会引起一系列人类和动物疾病,其中一些疾病危及生命,但目前还没有获得许可的抗肠道病毒药物。然而,苯磺酰胺衍生物和相关化合物最近已被证明可以通过在许多肠道病毒中保守的带正电荷的表面凹陷处结合衣壳来阻止一系列肠道病毒的感染。还确定谷胱甘肽对于许多肠道病毒的组装至关重要,它与衣壳蛋白相互作用以促进五聚体组装中间体的形成,尽管其机制尚不清楚。在这里,我们通过肠道病毒 F3 的高分辨率结构分析表明,还原型谷胱甘肽与苯磺酰胺衍生物结合到相同的原体间口袋。结合的谷胱甘肽与邻近的原聚体产生强烈的相互作用,从而解释了这种可成药结合袋的潜在生物学作用,并描绘了潜在抗病毒药物的药效团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号