当前位置:
X-MOL 学术
›
Mol. Psychiatry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders.
Molecular Psychiatry ( IF 9.6 ) Pub Date : 2020-01-03 , DOI: 10.1038/s41380-019-0639-2 Michael Notaras 1 , Maarten van den Buuse 2, 3, 4
Molecular Psychiatry ( IF 9.6 ) Pub Date : 2020-01-03 , DOI: 10.1038/s41380-019-0639-2 Michael Notaras 1 , Maarten van den Buuse 2, 3, 4
Affiliation
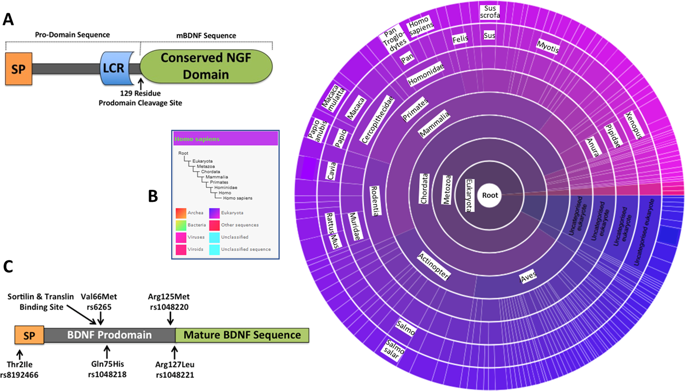
|
Brain-derived neurotrophic factor (BDNF) is widely accepted for its involvement in resilience and antidepressant drug action, is a common genetic locus of risk for mental illnesses, and remains one of the most prominently studied molecules within psychiatry. Stress, which arguably remains the "lowest common denominator" risk factor for several mental illnesses, targets BDNF in disease-implicated brain regions and circuits. Altered stress-related responses have also been observed in animal models of BDNF deficiency in vivo, and BDNF is a common downstream intermediary for environmental factors that potentiate anxiety- and depressive-like behavior. However, BDNF's broad functionality has manifested a heterogeneous literature; likely reflecting that BDNF plays a hitherto under-recognized multifactorial role as both a regulator and target of stress hormone signaling within the brain. The role of BDNF in vulnerability to stress and stress-related disorders, such as posttraumatic stress disorder (PTSD), is a prominent example where inconsistent effects have emerged across numerous models, labs, and disciplines. In the current review we provide a contemporary update on the neurobiology of BDNF including new data from the behavioral neuroscience and neuropsychiatry literature on fear memory consolidation and extinction, stress, and PTSD. First we present an overview of recent advances in knowledge on the role of BDNF within the fear circuitry, as well as address mounting evidence whereby stress hormones interact with endogenous BDNF-TrkB signaling to alter brain homeostasis. Glucocorticoid signaling also acutely recruits BDNF to enhance the expression of fear memory. We then include observations that the functional common BDNF Val66Met polymorphism modulates stress susceptibility as well as stress-related and stress-inducible neuropsychiatric endophenotypes in both man and mouse. We conclude by proposing a BDNF stress-sensitivity hypothesis, which posits that disruption of endogenous BDNF activity by common factors (such as the BDNF Val66Met variant) potentiates sensitivity to stress and, by extension, vulnerability to stress-inducible illnesses. Thus, BDNF may induce plasticity to deleteriously promote the encoding of fear and trauma but, conversely, also enable adaptive plasticity during extinction learning to suppress PTSD-like fear responses. Ergo regulators of BDNF availability, such as the Val66Met polymorphism, may orchestrate sensitivity to stress, trauma, and risk of stress-induced disorders such as PTSD. Given an increasing interest in personalized psychiatry and clinically complex cases, this model provides a framework from which to experimentally disentangle the causal actions of BDNF in stress responses, which likely interact to potentiate, produce, and impair treatment of, stress-related psychiatric disorders.
中文翻译:

BDNF 在恐惧记忆、压力敏感性和压力相关疾病中的神经生物学。
脑源性神经营养因子 (BDNF) 因其参与恢复力和抗抑郁药物作用而被广泛接受,是精神疾病风险的常见基因位点,并且仍然是精神病学中研究最突出的分子之一。压力可以说仍然是几种精神疾病的“最小公分母”风险因素,它针对与疾病有关的大脑区域和回路中的 BDNF。在体内 BDNF 缺乏的动物模型中也观察到改变的压力相关反应,并且 BDNF 是增强焦虑和抑郁样行为的环境因素的常见下游中介。然而,BDNF 的广泛功能已经表现出异构的文献。可能反映了 BDNF 作为大脑内应激激素信号传导的调节剂和靶点,发挥着迄今为止未被充分认识的多因素作用。BDNF 在易受压力和压力相关疾病(如创伤后应激障碍 (PTSD) 等)易感性中的作用是一个突出的例子,其中在众多模型、实验室和学科中出现了不一致的影响。在当前的评论中,我们提供了 BDNF 神经生物学的当代更新,包括来自行为神经科学和神经精神病学文献中关于恐惧记忆巩固和消退、压力和 PTSD 的新数据。首先,我们概述了关于 BDNF 在恐惧回路中的作用的最新进展,并提出了越来越多的证据,即压力荷尔蒙与内源性 BDNF-TrkB 信号传导相互作用以改变大脑稳态。糖皮质激素信号传导也急剧募集 BDNF 以增强恐惧记忆的表达。然后,我们观察到功能性常见的 BDNF Val66Met 多态性调节人和小鼠的压力易感性以及压力相关和压力诱导的神经精神内表型。我们通过提出 BDNF 压力敏感性假设得出结论,该假设假设共同因素(例如 BDNF Val66Met 变体)破坏内源性 BDNF 活性会增强对压力的敏感性,进而增强对压力诱发疾病的脆弱性。因此,BDNF 可能会诱导可塑性,从而有害地促进恐惧和创伤的编码,但相反,它也会在消退学习期间启用适应性可塑性,以抑制类似 PTSD 的恐惧反应。BDNF 可用性的尔格调节剂,例如 Val66Met 多态性,可能协调对压力、创伤和压力诱发疾病(如 PTSD)风险的敏感性。鉴于对个性化精神病学和临床复杂病例的兴趣日益增加,该模型提供了一个框架,可以从实验上解开 BDNF 在压力反应中的因果作用,这可能相互作用以增强、产生和损害与压力相关的精神疾病的治疗。
更新日期:2020-01-04
中文翻译:

BDNF 在恐惧记忆、压力敏感性和压力相关疾病中的神经生物学。
脑源性神经营养因子 (BDNF) 因其参与恢复力和抗抑郁药物作用而被广泛接受,是精神疾病风险的常见基因位点,并且仍然是精神病学中研究最突出的分子之一。压力可以说仍然是几种精神疾病的“最小公分母”风险因素,它针对与疾病有关的大脑区域和回路中的 BDNF。在体内 BDNF 缺乏的动物模型中也观察到改变的压力相关反应,并且 BDNF 是增强焦虑和抑郁样行为的环境因素的常见下游中介。然而,BDNF 的广泛功能已经表现出异构的文献。可能反映了 BDNF 作为大脑内应激激素信号传导的调节剂和靶点,发挥着迄今为止未被充分认识的多因素作用。BDNF 在易受压力和压力相关疾病(如创伤后应激障碍 (PTSD) 等)易感性中的作用是一个突出的例子,其中在众多模型、实验室和学科中出现了不一致的影响。在当前的评论中,我们提供了 BDNF 神经生物学的当代更新,包括来自行为神经科学和神经精神病学文献中关于恐惧记忆巩固和消退、压力和 PTSD 的新数据。首先,我们概述了关于 BDNF 在恐惧回路中的作用的最新进展,并提出了越来越多的证据,即压力荷尔蒙与内源性 BDNF-TrkB 信号传导相互作用以改变大脑稳态。糖皮质激素信号传导也急剧募集 BDNF 以增强恐惧记忆的表达。然后,我们观察到功能性常见的 BDNF Val66Met 多态性调节人和小鼠的压力易感性以及压力相关和压力诱导的神经精神内表型。我们通过提出 BDNF 压力敏感性假设得出结论,该假设假设共同因素(例如 BDNF Val66Met 变体)破坏内源性 BDNF 活性会增强对压力的敏感性,进而增强对压力诱发疾病的脆弱性。因此,BDNF 可能会诱导可塑性,从而有害地促进恐惧和创伤的编码,但相反,它也会在消退学习期间启用适应性可塑性,以抑制类似 PTSD 的恐惧反应。BDNF 可用性的尔格调节剂,例如 Val66Met 多态性,可能协调对压力、创伤和压力诱发疾病(如 PTSD)风险的敏感性。鉴于对个性化精神病学和临床复杂病例的兴趣日益增加,该模型提供了一个框架,可以从实验上解开 BDNF 在压力反应中的因果作用,这可能相互作用以增强、产生和损害与压力相关的精神疾病的治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号