当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 3‐Keto Pyridines from the Conjugated Allenone – Alkynylamine Oxidative Cyclization Catalyzed by Supported Au Nanoparticles
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-16 , DOI: 10.1002/adsc.201901451 Michael Fragkiadakis 1 , Marios Kidonakis 1 , Leandros Zorba 1 , Manolis Stratakis 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-16 , DOI: 10.1002/adsc.201901451 Michael Fragkiadakis 1 , Marios Kidonakis 1 , Leandros Zorba 1 , Manolis Stratakis 1
Affiliation
|
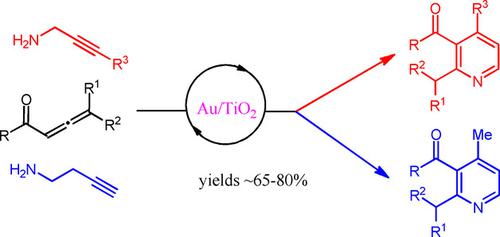
Recyclable supported Au nanoparticles on TiO2 catalyze the cyclization of N‐propargyl or N‐homopropargyl β‐enaminones followed by dehydrogenation (aromatization) leading to substituted 3‐keto pyridines or 4‐picolines in very good yields. This pathway is in contrast to their known cyclization in the presence of Au(I) or Au(III) catalysts which provides 1,4‐oxazepines, instead. The enaminones are formed in situ upon mixing a conjugated allenone or allenyl ester with the alkynylamine, thus the pyridine‐forming transformation is typically a one pot process.
中文翻译:

负载金纳米粒子催化共轭的烯丙酮-炔胺氧化环化反应合成3-酮吡啶
TiO 2上的可回收负载型Au纳米颗粒催化N-炔丙基或N-同炔丙基β-烯胺酮的环化,然后进行脱氢(芳构化),从而以非常好的收率产生取代的3-酮吡啶或4-甲基吡啶。该途径与已知在Au(I)或Au(III)催化剂存在下环化的相反,后者提供1,4-氧杂氮杂pine。烯胺酮是在将共轭的Allenone或Allenyl酯与炔基胺混合后原位形成的,因此形成吡啶的转化通常是一锅法。
更新日期:2020-01-17
中文翻译:

负载金纳米粒子催化共轭的烯丙酮-炔胺氧化环化反应合成3-酮吡啶
TiO 2上的可回收负载型Au纳米颗粒催化N-炔丙基或N-同炔丙基β-烯胺酮的环化,然后进行脱氢(芳构化),从而以非常好的收率产生取代的3-酮吡啶或4-甲基吡啶。该途径与已知在Au(I)或Au(III)催化剂存在下环化的相反,后者提供1,4-氧杂氮杂pine。烯胺酮是在将共轭的Allenone或Allenyl酯与炔基胺混合后原位形成的,因此形成吡啶的转化通常是一锅法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号