当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two-year outcomes of infants enrolled in the first-in-human study of amnion cells for bronchopulmonary dysplasia.
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2019-11-27 , DOI: 10.1002/sctm.19-0251 Atul Malhotra 1, 2, 3 , Rebecca Lim 2, 4 , Joanne C Mockler 4 , Euan M Wallace 2, 4
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2019-11-27 , DOI: 10.1002/sctm.19-0251 Atul Malhotra 1, 2, 3 , Rebecca Lim 2, 4 , Joanne C Mockler 4 , Euan M Wallace 2, 4
Affiliation
|
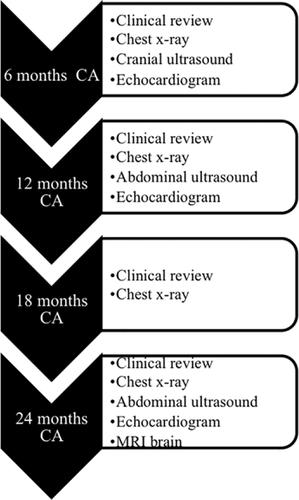
We previously reported on the immediate safety and neonatal outcomes of six premature infants with severe bronchopulmonary dysplasia (BPD) who were administered human amnion epithelial cells (hAECs). One infant died in the neonatal period due to unrelated causes. In this study, we aimed to assess the long‐term safety and follow‐up outcomes of the five surviving infants until 2 years corrected age (CA). hAECs were administered intravenously at a dose of 1 × 106 cells per kilogram after 36 weeks postconceptional age in infants with established BPD. Study follow‐up consisted of assessment of any adverse events, growth, and respiratory, cardiac, and neurodevelopmental outcomes over four time points (6, 12, 18, and 24 months CA). Investigations included chest x‐rays, cranial and abdominal ultrasounds, and echocardiograms at regular intervals as well as a magnetic resonance imaging (MRI) brain at 2 years CA. All five infants were alive at 2 years CA. Median time to wean off oxygen was 24 (10‐36) months. Two infants had pulmonary hypertension, which resolved by 2 years of age. Four infants were rehospitalized briefly for viral or bacterial infections during the 2 years. MRI brain findings included normal (n = 1), and mild to moderate white matter loss (n = 2). Neurodisabilities diagnosed included hemiplegic cerebral palsy (n = 1), global developmental delay (n = 3), and severe hearing loss (n = 3). No evidence of tumor formation was noted on physical examinations or on any imaging. There were no long‐term adverse events observed that could be attributed to hAEC administration. We observed long‐term effects of extreme prematurity and severe BPD in the cohort.
中文翻译:

婴儿的两年结局参与了针对支气管肺发育不良的羊膜细胞的首次人体研究。
我们之前曾报道过六个严重的支气管肺发育不良(BPD)早产儿的人羊膜上皮细胞(hAEC)的即时安全性和新生儿结局。一名婴儿由于不相关的原因在新生儿期死亡。在这项研究中,我们旨在评估5名存活婴儿的长期安全性和随访结果,直至2岁校正年龄(CA)。hAEC以1×10 6的剂量静脉内给药出生后BPD的婴儿在受孕后36周后每公斤可吸收的细胞数。研究随访包括评估四个时间点(CA 6、12、18和24个月)的任何不良事件,生长以及呼吸,心脏和神经发育结果。研究包括定期进行胸部X线检查,颅骨和腹部超声检查以及超声心动图,以及在CA 2年时的磁共振成像(MRI)脑。所有五个婴儿在CA 2岁时仍存活。断氧的中位时间为24(10-36)个月。两名婴儿患有肺动脉高压,可在2岁时消退。在这2年中,有4名婴儿因病毒或细菌感染而短暂住院。MRI脑部检查结果包括正常(n = 1)和轻度至中度白质流失(n = 2)。诊断出的神经残疾包括偏瘫性脑瘫(n = 1),整体发育迟缓(n = 3)和严重的听力丧失(n = 3)。体格检查或任何影像学检查均未发现肿瘤形成的证据。没有观察到可归因于hAEC给药的长期不良事件。我们观察到队列中极端早产和严重BPD的长期影响。
更新日期:2019-11-27
中文翻译:

婴儿的两年结局参与了针对支气管肺发育不良的羊膜细胞的首次人体研究。
我们之前曾报道过六个严重的支气管肺发育不良(BPD)早产儿的人羊膜上皮细胞(hAEC)的即时安全性和新生儿结局。一名婴儿由于不相关的原因在新生儿期死亡。在这项研究中,我们旨在评估5名存活婴儿的长期安全性和随访结果,直至2岁校正年龄(CA)。hAEC以1×10 6的剂量静脉内给药出生后BPD的婴儿在受孕后36周后每公斤可吸收的细胞数。研究随访包括评估四个时间点(CA 6、12、18和24个月)的任何不良事件,生长以及呼吸,心脏和神经发育结果。研究包括定期进行胸部X线检查,颅骨和腹部超声检查以及超声心动图,以及在CA 2年时的磁共振成像(MRI)脑。所有五个婴儿在CA 2岁时仍存活。断氧的中位时间为24(10-36)个月。两名婴儿患有肺动脉高压,可在2岁时消退。在这2年中,有4名婴儿因病毒或细菌感染而短暂住院。MRI脑部检查结果包括正常(n = 1)和轻度至中度白质流失(n = 2)。诊断出的神经残疾包括偏瘫性脑瘫(n = 1),整体发育迟缓(n = 3)和严重的听力丧失(n = 3)。体格检查或任何影像学检查均未发现肿瘤形成的证据。没有观察到可归因于hAEC给药的长期不良事件。我们观察到队列中极端早产和严重BPD的长期影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号