Current Pharmaceutical Biotechnology ( IF 2.2 ) Pub Date : 2020-06-30 , DOI: 10.2174/1389201020666191024172425 Kiyoshi Takasuna 1 , Katsuyuki Kazusa 2 , Tomohiro Hayakawa 2
|
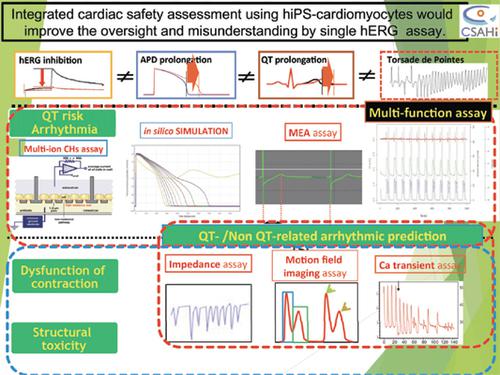
Current cardiac safety assessment platforms (in vitro hERG-centric, APD, and/or in vivo animal QT assays) are not fully predictive of drug-induced Torsades de Pointes (TdP) and do not address other mechanism-based arrhythmia, including ventricular tachycardia or ventricular fibrillation, or cardiac safety liabilities such as contractile and structural cardiotoxicity which are another growing safety concerns. We organized the Consortium for Safety Assessment using Human iPS cells (CSAHi; http://csahi.org/en/) in 2013, based on the Japan Pharmaceutical Manufacturers Association (JPMA), to verify the application of human iPS/ES cell-derived cardiomyocytes for drug safety evaluation. The CSAHi HEART team focused on comprehensive screening strategies to predict a diverse range of cardiotoxicities using recently introduced platforms such as the Multi-Electrode Array (MEA), cellular impedance, Motion Field Imaging (MFI), and optical imaging of Ca transient to identify strengths and weaknesses of each platform. Our study showed that hiPS-CMs used in these platforms could detect pharmacological responses that were more relevant to humans compared to existing hERG, APD, or Langendorff (MAPD/contraction) assays. Further, MEA and other methods such as impedance, MFI, and Ca transient assays provided paradigm changes of platforms for predicting drug-induced QT risk and/or arrhythmia or contractile dysfunctions. In contrast, since discordances such as overestimation (false positive) of arrhythmogenicity, oversight, or opposite conclusions in positive inotropic and negative chronotropic activities to some compounds were also confirmed, possibly due to their functional immaturity of hiPS-CMs, hiPS-CMs should be used in these platforms for cardiac safety assessment based upon their advantages and disadvantages.
中文翻译:

使用hiPS心肌细胞进行全面的心脏安全评估(使用人iPS细胞进行安全评估的联盟:CSAHi)。
当前的心脏安全评估平台(以体外hERG为中心的,APD和/或体内动物QT分析)不能完全预测药物诱发的扭转型扭转性室性心律失常(TdP),并且不能解决其他基于机制的心律失常,包括室性心动过速或心室纤颤,或心脏安全性责任,例如收缩性和结构性心脏毒性,这是另一个日益受到关注的安全性问题。我们在2013年根据日本制药厂商协会(JPMA)成立了使用人类iPS细胞的安全性评估联盟(CSAHi; http://csahi.org/en/),以验证人类iPS / ES细胞的应用衍生的心肌细胞用于药物安全性评估。CSAHi HEART团队专注于综合筛选策略,可使用最近推出的平台(例如多电极阵列(MEA),细胞阻抗,运动场成像(MFI)和Ca瞬变的光学成像)来预测各种心脏毒性,从而确定强度和每个平台的弱点。我们的研究表明,与现有的hERG,APD或Langendorff(MAPD / contract)分析相比,在这些平台中使用的hiPS-CMs可以检测到与人类更相关的药理反应。此外,MEA和其他方法(例如阻抗,MFI和Ca瞬态分析)为预测药物诱发的QT风险和/或心律不齐或收缩功能障碍提供了平台的范式变化。相反,由于心律失常的高估(误报),监督等不一致,











































 京公网安备 11010802027423号
京公网安备 11010802027423号