Protein & Peptide Letters ( IF 1.0 ) Pub Date : 2020-05-31 , DOI: 10.2174/0929866526666191113151934 Hikaru Nakazawa 1 , Mitsuo Umetsu 1 , Tatsuya Hirose 1 , Takamitsu Hattori 1 , Izumi Kumagai 1
|
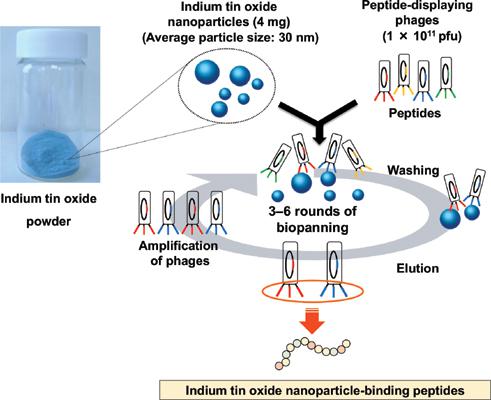
Background: By recent advances in phage-display approaches, many oligopeptides exhibiting binding affinities for metal oxides have been identified. Indium tin oxide is one of the most widely used conductive oxides, because it has a large band gap of 3.7–4.0 eV. In recent years, there have been reports about several ITO-based biosensors. Development of an ITO binding interface for the clustering of sensor proteins without complex bioconjugates is required.
Objective: In this article, we aimed to identify peptides that bind to indium tin oxide nanoparticles via different binding mechanisms.
Methods: Indium tin oxide nanoparticles binding peptide ware selected using phage display and biopanning against indium tin oxide, under five different buffer conditions and these peptides characterized about binding affinity and specificity.
Results: Three types of indium tin oxide nanoparticles-binding peptides were selected from 10 types of peptide candidates identified in phage display and biopanning. These included ITOBP8, which had an acidic isoelectric point, and was identified when a buffer containing guanidine was used, and ITOBP6 and ITOBP7, which contained a His-His-Lys sequence at their N-termini, and were identified when a highly concentrated phosphate elution buffer with a low ionic strength was used. Among these peptides, ITOBP6 exhibited the strongest indium tin oxide nanoparticlesbinding affinity (dissociation constant, 585 nmol/L; amount of protein bound at saturation, 17.5 nmol/m 2 - particles).
Conclusion: These results indicate that peptides with specific binding properties can be obtained through careful selection of the buffer conditions in which the biopanning procedure is performed.
中文翻译:

在各种缓冲液条件下通过噬菌体展示和生物淘选鉴定氧化铟锡纳米粒子结合肽。
背景:通过噬菌体展示方法的最新进展,已鉴定出许多对金属氧化物表现出结合亲和力的寡肽。铟锡氧化物是使用最广泛的导电氧化物之一,因为它具有3.7-4.0 eV的大带隙。近年来,已有关于几种基于ITO的生物传感器的报道。需要开发用于没有复杂生物缀合物的传感器蛋白聚类的ITO结合界面。
目的:在本文中,我们旨在鉴定通过不同结合机制与铟锡氧化物纳米粒子结合的肽。
方法:在五个不同的缓冲液条件下,通过噬菌体展示和针对氧化铟锡的生物淘选来选择氧化铟锡纳米粒子结合肽,这些肽的特征在于结合亲和力和特异性。
结果:从噬菌体展示和生物淘选中鉴定的10种候选肽中选择了三种类型的氧化铟锡纳米粒子结合肽。其中包括具有酸性等电点的ITOBP8,并在使用含胍的缓冲液时被鉴定;在其N末端包含His-His-Lys序列的ITOBP6和ITOBP7,以及在高浓度磷酸盐时被鉴定的ITOBP6和ITOBP7。使用低离子强度的洗脱缓冲液。在这些肽中,ITOBP6表现出最强的铟锡氧化物纳米颗粒结合亲和力(解离常数,585 nmol / L;饱和结合蛋白的量,17.5 nmol / m 2-颗粒)。
结论:这些结果表明,通过仔细选择进行生物淘选过程的缓冲液条件,可以获得具有特异性结合特性的肽。









































 京公网安备 11010802027423号
京公网安备 11010802027423号