Current Pharmaceutical Biotechnology ( IF 2.2 ) Pub Date : 2020-06-30 , DOI: 10.2174/1389201020666190628143345 Ayano Satsuka 1 , Yasunari Kanda 1
|
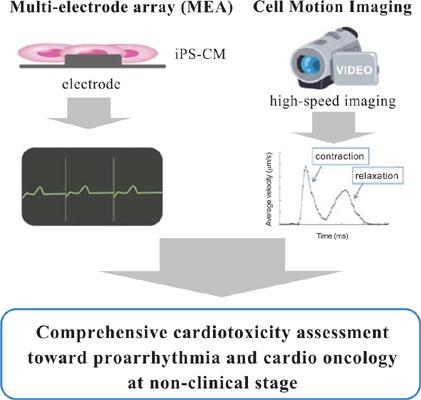
Growing evidence suggests that Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs) can be used as a new human cell-based platform to assess cardiac toxicity/safety during drug development. Cardiotoxicity assessment is highly challenging due to species differences and various toxicities, such as electrophysiological and contractile toxicities, which can result in proarrhythmia and heart failure. To explore proarrhythmic risk, the Multi-Electrode Array (MEA) platform is widely used to assess QT-interval prolongation and the proarrhythmic potential of drug candidates using hiPSC-CMs. Several consortiums, including the Comprehensive in vitro Proarrhythmia Assay (CiPA) and the Japanese iPS Cardiac Safety Assessment (JiCSA), have demonstrated the applicability of hiPSC-CMs/MEA for assessing the torsadogenic potential of drug candidates. Additionally, contractility is a key safety issue in drug development, and efforts have been undertaken to measure contractility by a variety of imaging-based methods using iPS-CMs. Therefore, hiPSC-CMs might represent a standard testing tool for evaluating the proarrhythmic and contractile potentials. This review provides new insights into the practical application of hiPSC-CMs in early or late-stage nonclinical testing during drug development.
中文翻译:

使用人iPS细胞衍生的心肌细胞对药物的心脏毒性评估:走向心律失常风险和心脏肿瘤学。
越来越多的证据表明,人诱导的多能干细胞衍生的心肌细胞(hiPSC-CM)可以用作评估药物开发过程中心脏毒性/安全性的新的基于人细胞的平台。由于物种差异和各种毒性(例如电生理和收缩毒性),心脏毒性评估极具挑战性,这可能导致心律失常和心力衰竭。为了探索心律失常的风险,多电极阵列(MEA)平台被广泛用于评估使用hiPSC-CM的QT间隔延长和候选药物的心律失常潜力。包括国际体外心律失常综合检测(CiPA)和日本iPS心脏安全性评估(JiCSA)在内的多个联盟,已经证明了hiPSC-CMs / MEA在评估候选药物的致畸潜力方面的适用性。另外,可收缩性是药物开发中的关键安全问题,并且已经努力使用iPS-CM通过各种基于成像的方法来测量可收缩性。因此,hiPSC-CM可能代表评估心律失常和收缩潜力的标准测试工具。这篇综述提供了hiPSC-CM在药物开发过程中早期或晚期非临床测试中的实际应用的新见解。hiPSC-CM可能代表评估心律失常和收缩潜力的标准测试工具。这篇综述提供了hiPSC-CM在药物开发过程中早期或晚期非临床测试中的实际应用的新见解。hiPSC-CM可能代表评估心律失常和收缩潜力的标准测试工具。这篇综述提供了hiPSC-CM在药物开发过程中早期或晚期非临床测试中的实际应用的新见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号