Current Protein & Peptide Science ( IF 1.9 ) Pub Date : 2020-08-31 , DOI: 10.2174/1389203720666190618095719 Shahnawaz Rehman 1 , Mohammad Faisal 2 , Abdulrahman A Alatar 2 , Saheem Ahmad 1
|
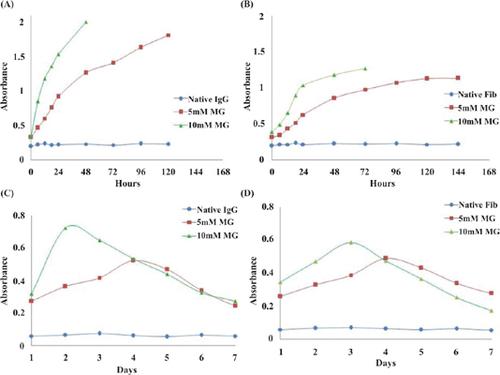
Background: Non-enzymatic glycation of proteins plays a significant role in the pathogenesis of secondary diabetic complications via the formation of advanced glycation end products (AGEs) and increased oxidative stress. Methylglyoxal (MG), a highly reactive dicarbonyl of class α-oxoaldehyde that generates during glucose oxidation and lipid peroxidation, contributes to glycation.
Objective: This comparative study focuses on methylglyoxal induced glycoxidative damage suffered by immunoglobulin G (IgG) and fibrinogen, and to unveil implication of structural modification of serum proteins in diabetes-associated secondary complications.
Methods: The methylglyoxal induced structural alterations in IgG and fibrinogen were analyzed by UVvis, fluorescence, circular dichroism and Fourier transform infrared (FT-IR) spectroscopy. Ketoamine moieties, carbonyl contents, 5-Hydroxymethylfurfural (HMF) and malondyaldehyde were also quantified. Free lysine and arginine estimation, detection of non-fluorogenic carboxymethyllysine (CML) and fibril formation were confirmed by thioflavin T (ThT) assay.
Results: Structural alterations, increased carbonyl contents and ketoamines were reported in MG glycated IgG and fibrinogen against their native analogues.
Conclusion: The experiment results validate structural modifications, increased oxidative stress and AGEs formation. Thus, we can conclude that IgG-AGEs and Fib-AGEs formed during MG induced glycation of IgG and fibrinogen could impede normal physiology and might initiates secondary complications in diabetic patients.
中文翻译:

反应性羰基物质甲基乙二醛在血清蛋白、免疫球蛋白-G 和纤维蛋白原中诱导的物理化学变化。
背景:蛋白质的非酶糖化通过晚期糖基化终产物 (AGEs) 的形成和氧化应激的增加,在继发性糖尿病并发症的发病机制中发挥着重要作用。甲基乙二醛 (MG) 是一种高活性的 α-氧醛类二羰基,在葡萄糖氧化和脂质过氧化过程中产生,有助于糖化。
目的:这项比较研究的重点是甲基乙二醛诱导的免疫球蛋白 G (IgG) 和纤维蛋白原遭受的糖氧化损伤,并揭示血清蛋白结构修饰在糖尿病相关继发性并发症中的意义。
方法:通过紫外可见光、荧光、圆二色性和傅里叶变换红外 (FT-IR) 光谱分析甲基乙二醛引起的 IgG 和纤维蛋白原的结构改变。酮胺部分、羰基含量、5-羟甲基糠醛 (HMF) 和丙二醛也被量化。游离赖氨酸和精氨酸的估计、非荧光羧甲基赖氨酸 (CML) 的检测和原纤维形成均通过硫代黄素 T (ThT) 测定证实。
结果:据报道,相对于天然类似物,MG 糖化 IgG 和纤维蛋白原的结构改变、羰基含量和酮胺含量增加。
结论:实验结果证实了结构改变、氧化应激增加和 AGEs 形成。因此,我们可以得出结论,在 MG 诱导的 IgG 和纤维蛋白原糖化过程中形成的 IgG-AGEs 和 Fib-AGEs 可能会阻碍正常的生理机能,并可能引发糖尿病患者的继发性并发症。










































 京公网安备 11010802027423号
京公网安备 11010802027423号